26.3 OTHER BIOGEOCHEMICAL CYCLES
Let’s think further about prokaryotic metabolism. Anoxygenic photosynthetic bacteria commonly use reduced sulfur compounds as electron donors for photosynthesis (instead of water), and bacterial heterotrophs often use oxidized sulfur compounds as electron acceptors in anaerobic respiration (instead of oxygen gas). By reducing oxidized sulfur compounds in the course of anaerobic respiration, microorganisms generate the reduced sulfur compounds required for anoxygenic photosynthesis. The reverse is also true: Oxidation of sulfur compounds during anoxygenic photosynthesis generates the oxidized sulfur molecules used in anaerobic respiration.
Once again, we see complementary metabolic pathways. The expanded carbon cycle of Bacteria and Archaea also promotes the cycling of sulfur, nitrogen, and other biologically important elements.
26.3.1 Bacteria and archaeons dominate Earth’s sulfur cycle.
Our bodies are mostly carbon, oxygen, and hydrogen. We also contain about 0.2% sulfur by weight. Sulfur is a component of the amino acids cysteine and methionine and therefore is present in many proteins (Chapter 2). Sulfur is also present in iron–sulfur clusters that are key components of enzymes fundamental to electron transfer. It occurs, as well, in some membranes and is found in a number of vitamins and other cofactors, including coenzyme A, thiamine (vitamin B1), and biotin. Where do we get the sulfur we need? From the food we eat—protein in the steak or fish on our dinner plate provides a rich source of sulfur. Where do cows and fish get the sulfur they need? Like us, from the food they eat. Food chains don’t go on forever, so somewhere in the system, some organisms must take up inorganic sulfur from the environment. Primary producers, organisms that reduce CO2 to form carbohydrates, fill this role.
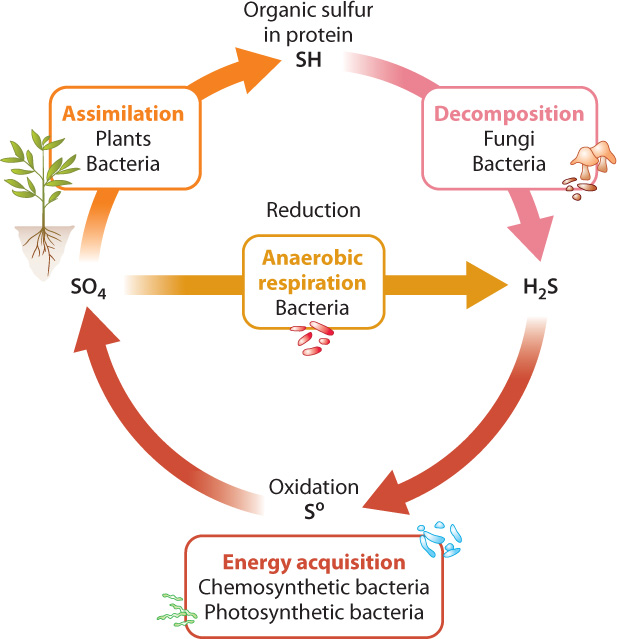
On land, plants take up sulfate (SO42−) ions from the soil and reduce them within their cells to hydrogen sulfide (H2S) that can be incorporated into cysteine and other biomolecules (Fig. 26.10). This process is called assimilation. Algae and photosynthetic bacteria do much the same thing in lakes, rivers, and oceans. Why don’t primary producers take up H2S directly? There are two answers to this question. First, H2S is rapidly oxidized in the presence of oxygen and so does not occur in environments where oxygenic photosynthesis is common. Second, H2S is generally toxic to eukaryotic organisms, so plants and algae do not thrive where it is abundant. (The H2S produced within eukaryotic cells has a short lifetime and is restricted to intracellular sites distant from those that are vulnerable to H2S.)
We’ve now seen half the sulfur cycle, the conversion of sulfate to H2S within cells. How did sulfate molecules get into the soil in the first place, and what happens to biological sulfur compounds when cells die? After death, fungi and bacteria decompose cells, returning carbon, sulfur, and other compounds to the environment. Reduced sulfur compounds released from decomposing cells are then oxidized by bacteria and archaeons, completing the cycle (Fig. 26.10).
How do those reduced sulfur compounds get oxidized? There are two pathways, both of which involve microorganisms (Fig. 26.10). Chemoautotrophic bacteria react H2S with O2 to obtain energy, producing sulfate in the process. And in anoxygenic photosynthesis, bacteria use electrons from H2S to form carbohydrates from CO2, again generating sulfate as a by-product. The sulfate produced by these processes is consumed by heterotrophic bacteria living in oxygen-free environments. Sulfate rather than oxygen is used as the final electron acceptor in respiration and is reduced to H2S. In fact, most of the sulfur cycled biologically is used to drive energy metabolism in oxygen-poor environments, not to build proteins.
Note that eukaryotes use neither H2S for photo- (or chemo-) synthesis nor sulfate in respiration. Thus, the biological sulfur cycle is completed by bacteria and archaeons alone.
26.3.2 The nitrogen cycle is also driven by bacteria and archaeons.
The model provided by the sulfur cycle can be extended to other biologically important elements. Nitrogen is the fourth most abundant element in the human body, contributing 4% by weight as a component of proteins, nucleic acids, and other compounds. As was true of sulfur, primary producers incorporate inorganic forms of nitrogen into biomolecules, and we get the nitrogen we need from our food. As we’ll see, however, there is a twist.
26-11
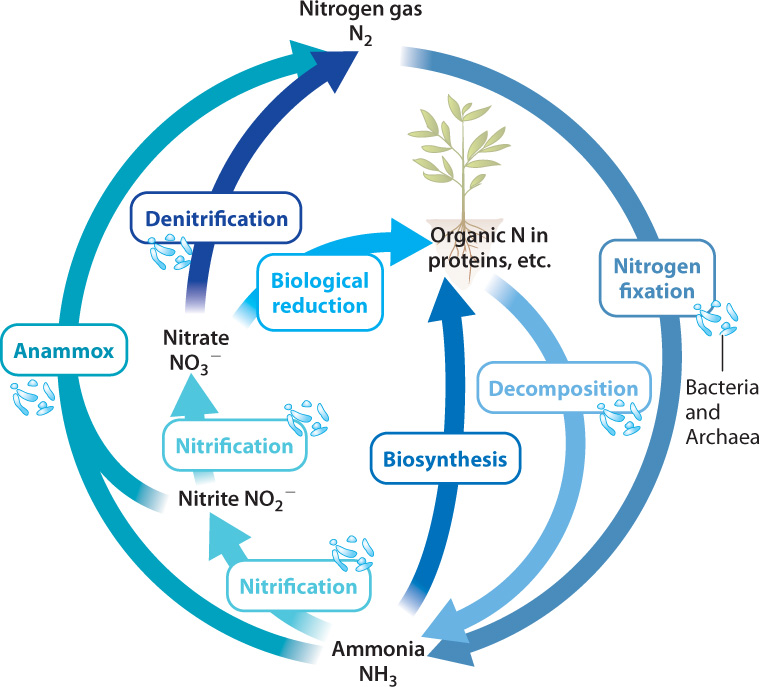
Let’s walk through the biological nitrogen cycle (Fig. 26.11). Carbon is a minor component of the atmosphere and sulfur only a trace constituent, but the air we breathe consists mostly (79%) of nitrogen gas (N2). Indeed, most of the nitrogen at Earth’s surface resides in the atmosphere. Although nitrogen is plentiful, the nitrogen available to primary producers in many environments is limited. Why should this be? The simple answer is that plants cannot make use of nitrogen gas and neither can algae. Only certain bacteria and archaeons can reduce N2 to ammonia (NH3), a form of nitrogen that can be incorporated into biomolecules.
The process of converting N2 into a biologically useful form such as ammonia is called nitrogen fixation, one of the most important reactions ever evolved by organisms. Farmers plant soybeans to regenerate nitrogen nutrients in soils, but it is not the soybean that fixes nitrogen. Rather, soybean roots have nodules that harbor nitrogen-fixing bacteria (Fig. 26.12). That is, plants like soybean have entered into an intimate partnership with nitrogen-fixing bacteria to obtain the biologically useful forms of nitrogen needed for growth.
The nitrogen cycle, then, begins with nitrogen fixation, and it continues through many additional metabolisms, most of them confined to bacteria and archaeons. Ammonia released by the decomposition of dead cells can be taken up by primary producers, but commonly it is oxidized to nitrate (NO3−) by chemoautotrophic bacteria, a process called nitrification (see Fig. 26.11). This process works efficiently in both soil and water, and so nitrate is the most common form of nitrogen available to life in both environments.

Anaerobic respiration completes the cycle by returning N2 to the atmosphere. Some bacteria can use nitrate as an electron acceptor in respiration, a process known as denitrification (see Fig. 26.11). Because denitrification is so efficient at returning nitrogen to the atmosphere as nitrogen gas, nitrogen fixation must continually make nitrogen available for organisms. Without nitrogen fixation, the nitrogen cycle would slowly wind down, and the productivity of Earth’s biota—that is, of all its organisms—would wind down with it. This is why nitrogen fixation is such an important process.
Until the 1990s, the nitrogen cycle was described—completely, it was thought—in terms of the metabolic reactions just discussed. Then, a new and unexpected form of nitrogen metabolism was discovered in the oceans. A number of prokaryotic microorganisms function as chemoautotrophs by reacting ammonium ion (NH4+) with nitrite (NO2−) to gain energy. The reaction is shown here:
NH4+ + NO2− → N2 + 2H2O
26-12
Note that this is a redox reaction, like other reactions involved in energy metabolism. In this case, the ammonium ion is oxidized to nitrogen gas, and nitrite is reduced to nitrogen gas, with water produced as a by-product. The reaction, called anammox (anaerobic ammonia oxidation), provides only a small amount of energy, but it supports many bacteria and archaeons in oxygen-depleted parts of the ocean, providing a major route by which fixed nitrogen returns to the atmosphere as N2 (see Fig. 26.11).
The biological nitrogen cycle, then, begins and ends with prokaryotic metabolisms. Bacteria and archaeons fix N2 into a biologically available form, nitrifying bacteria oxidize ammonia to nitrate, and still other prokaryotes gain energy from denitrification and anammox, returning N2 to the air. Unlike the carbon and sulfur cycles, the nitrogen cycle does not rely at any point on elements stored as minerals in rocks and sediments. Most of Earth’s nitrogen was released to the atmosphere as gas early in our planet’s history, and so movement into sediments and out of volcanoes contributes relatively little to the cycle. Humans, however, contribute in important ways, most notably through the industrial synthesis of nitrate fertilizer (Chapter 48).