27.2 EUKARYOTIC ORIGINS
In the preceding section, we reviewed the basic features of eukaryotic cells. The distinctive cellular organization of Eukarya differs markedly from the simpler cells of Bacteria and Archaea. How did eukaryotes come to form such a remarkably diverse branch of the tree of life? In Case 5: The Human Microbiome, we saw that symbioses are common, even in humans. Is the relationship between humans and bacteria unusual, or are symbioses so fundamental that they played key roles in the evolution of the eukaryotic cell?
27.2.1 What role did symbiosis play in the origin of chloroplasts?
Case 5 The Human Microbiome: Diversity Within
The chloroplasts found in plant cells closely resemble certain photosynthetic bacteria, specifically cyanobacteria. The molecular workings of photosynthesis are nearly identical in the two, and the way that internal membranes organize the photosynthetic machinery of cyanobacteria closely resembles the way that the thylakoid membranes organize the photosynthetic machinery of chloroplasts (Chapter 8). The Russian botanist Konstantin Sergeevich Merezhkovsky recognized this similarity more than a century ago. He also understood that corals and some other organisms harbor algae within their tissues as symbionts that aid the growth of their host. A symbiont is an organism that lives in closely evolved association with another species. This association, called symbiosis, is discussed in Chapter 47.
27-5
Putting these two observations together, Merezhkovsky came up with a radical hypothesis. Chloroplasts, he argued, originated as symbiotic cyanobacteria that through time became permanently incorporated into their hosts. Merezkhovsky’s hypothesis of chloroplast origin by endosymbiosis (a symbiosis in which one partner lives within the other) was difficult to test with the tools available in the early twentieth century, and his idea was dismissed, more neglected than disproved, by most biologists.
In 1967, American biologist Lynn Margulis resurrected the endosymbiotic hypothesis, supporting her arguments with new types of data made possible by the then-emerging techniques of cell and molecular biology (Fig. 27.4). Transmission electron microscopy showed that structural similarities between chloroplasts and cyanobacteria extend to the submicrometer level, for example in the organization of the photosynthetic membranes (Fig. 27.4a). It also became clear that the chloroplasts in red and green algae (and in land plants) are separated from the cytoplasm that surrounds them by two membranes. This is expected if a cyanobacterial cell had been engulfed by a eukaryotic cell. The inner membrane corresponds to the cell membrane of the cyanobacterium, and the outer one is part of the engulfing cell’s plasma membrane. In addition, the biochemistry of photosynthesis was found to be essentially the same in cyanobacteria and chloroplasts. Both use two linked photosystems, a common mechanism for extracting electrons from water, and the same reactions to reduce carbon dioxide (CO2) into organic matter (Chapter 8).
FIG. 27.4: What is the evolutionary origin of chloroplasts?
HYPOTHESIS Chloroplasts evolved from cyanobacteria living as endosymbionts within a eukaryotic cell.
OBSERVATION Chloroplasts and cyanobacteria have closely similar internal membranes that organize the light reactions of photosynthesis (Fig. 27.4a).
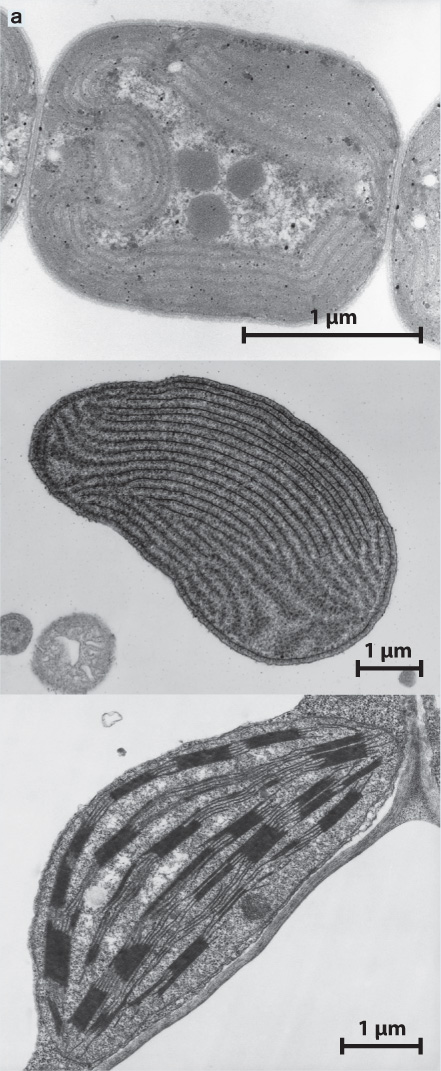
Transmission electron microscopy shows that cyanobacteria (top), red algal chloroplasts (middle), and plant chloroplasts (bottom) have very similar internal membranes that organize the light reactions of photosynthesis.
EXPERIMENT Like cyanobacteria, chloroplasts have DNA organized in a single circular chromosome. Phylogenies based on molecular sequence comparisons place chloroplasts among the cyanobacteria (Fig. 27.4b).
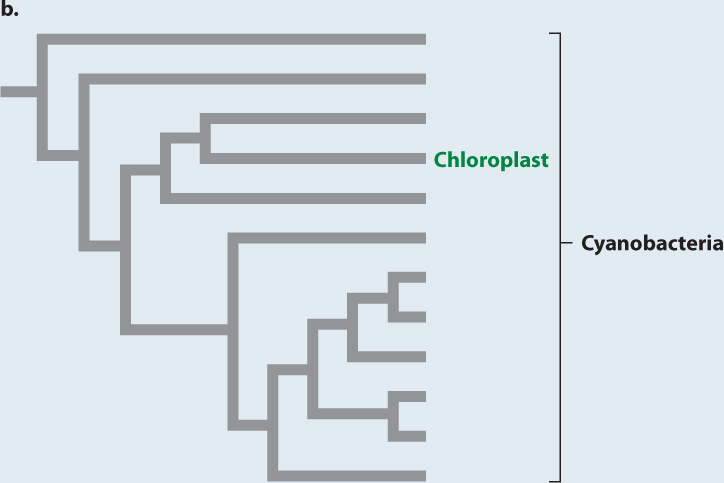
CONCLUSION Molecular and electron microscope data support the hypothesis that chloroplasts originated as endosymbiotic cyanobacteria.
SOURCE Giovannoni, S. J., S. Turner, G. J. Olsen, S. Barns, D. J. Lane, and N. R. Pace. 1988. “Evolutionary Relationships Among Cyanobacteria and Green Chloroplasts.” Journal of Bacteriology 170:3584–3592.
27-6
Such observations kindled renewed interest in the endosymbiotic hypothesis, but the decisive tests were made possible by another, and unexpected, discovery. Chloroplasts, it turns out, have their own DNA, organized into a single circular chromosome, like that of bacteria. Armed with this knowledge, investigators could use the tools of molecular sequence comparison (Chapter 23). The sequences of nucleotides in chloroplast genes closely match those of cyanobacterial genes but are strikingly different from sequences of genes in the nuclei of photosynthetic eukaryotes (Fig. 27.4b). This finding provides strong support for the hypothesis of Merezkhovsky and Margulis. Chloroplasts are indeed the descendants of symbiotic cyanobacteria that lived within eukaryotic cells.
Eukaryotes were able to acquire photosynthesis because they could engulf and retain cyanobacterial cells. But engulfing another microorganism was just the beginning. The cyanobacteria most closely related to chloroplasts have 2000 to 3000 genes. In contrast, among photosynthetic eukaryotes, chloroplast gene numbers vary from just 60 to 200. Where did the rest of the cyanobacterial genome go? Some genes may simply have been lost through evolution; unneeded chloroplast genes may have been lost if similar nuclear genes could supply chloroplast requirements. Many, however, were transported to the nucleus when chloroplasts broke or by hitching a ride with viruses. The nuclear genome of the flowering plant Arabidopsis contains several thousand genes of cyanobacterial origin. Some code for proteins destined for use within the chloroplast.
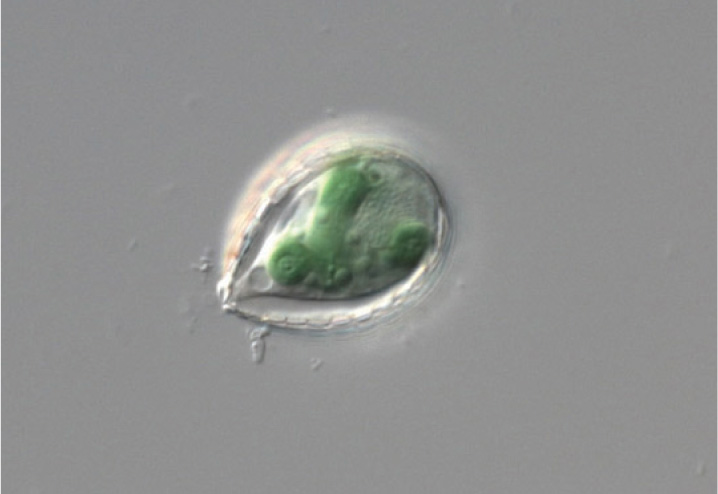
Although foreign to mammals and other vertebrate animals, symbiosis between a heterotrophic host and photosynthetic partner is, in fact, common throughout the eukaryotic domain. Reef corals, for example, harbor photosynthetic cells that live symbiotically within their tissues. Some corals have even lost the capacity to capture food from surrounding waters. Tridacna, the giant clam of tropical Pacific waters, obtains its nutrition from symbiotic algae that live within its tissues.
Until recently, most biologists agreed that chloroplasts originated from endosymbiotic cyanobacteria only once, in a common ancestor of the green algae and red algae. Now, remarkably, a second case of cyanobacterial endosymbiosis has come to light. Paulinella chromatophora is a photosynthetic amoeba (Fig. 27.5). Its chloroplast genes show that its chloroplast originated from a branch of the cyanobacteria different from and arising long after the one that gave rise to chloroplasts in other photosynthetic eukaryotes. About a third to a half of the ancestral genome remains in P. chromatophora chloroplasts, suggesting that this organism reflects chloroplast evolution still in progress.
27.2.2 What role did symbiosis play in the origin of mitochondria?
Case 5 The Human Microbiome: Diversity Within
Like chloroplasts, mitochondria closely resemble free-living bacteria in organization and biochemistry. And like chloroplasts, mitochondria contain DNA that confirms their close phylogenetic relationship to a form of bacteria—in this case proteobacteria (Chapter 26). Like chloroplasts, then, mitochondria originated as endosymbiotic bacteria.
Mitochondria are also like chloroplasts in having a small genome. Indeed, the mitochondrial genome is dramatically reduced compared to the ancestral proteobacterial genome, in most eukaryotes containing only a handful of functioning genes. Human mitochondria, for example, code for just 13 proteins and 24 RNAs. Once again, many genes from the original bacterial endosymbiont migrated to the nucleus, where they still reside.
Most eukaryotic cells contain mitochondria, but a few single-celled eukaryotic organisms found in oxygen-free environments do not. Biologists earlier hypothesized that these eukaryotes evolved before the endosymbiotic event that established mitochondria in cells having a nucleus, but that proposal turns out to be wrong. We know this because of the propensity for genes to migrate from the endosymbiont to the host’s nucleus. Every mitochondria-free eukaryote examined to date has relic mitochondrial genes in its nuclear genome, providing support for a second hypothesis, that eukaryotic cells without mitochondria had them once but have lost them. In fact, many eukaryotes that lack mitochondria contain small organelles called hydrogenosomes that generate ATP by anaerobic processes (Fig. 27.6). These organelles have little or no DNA, but genes of mitochondrial origin in the cells’ nuclei code for proteins that function in the hydrogenosome. Thus, hydrogenosomes appear to be highly altered mitochondria adapted to life in oxygen-poor environments. Other anaerobic eukaryotes contain still smaller organelles called mitosomes that also appear to be remnant mitochondria.
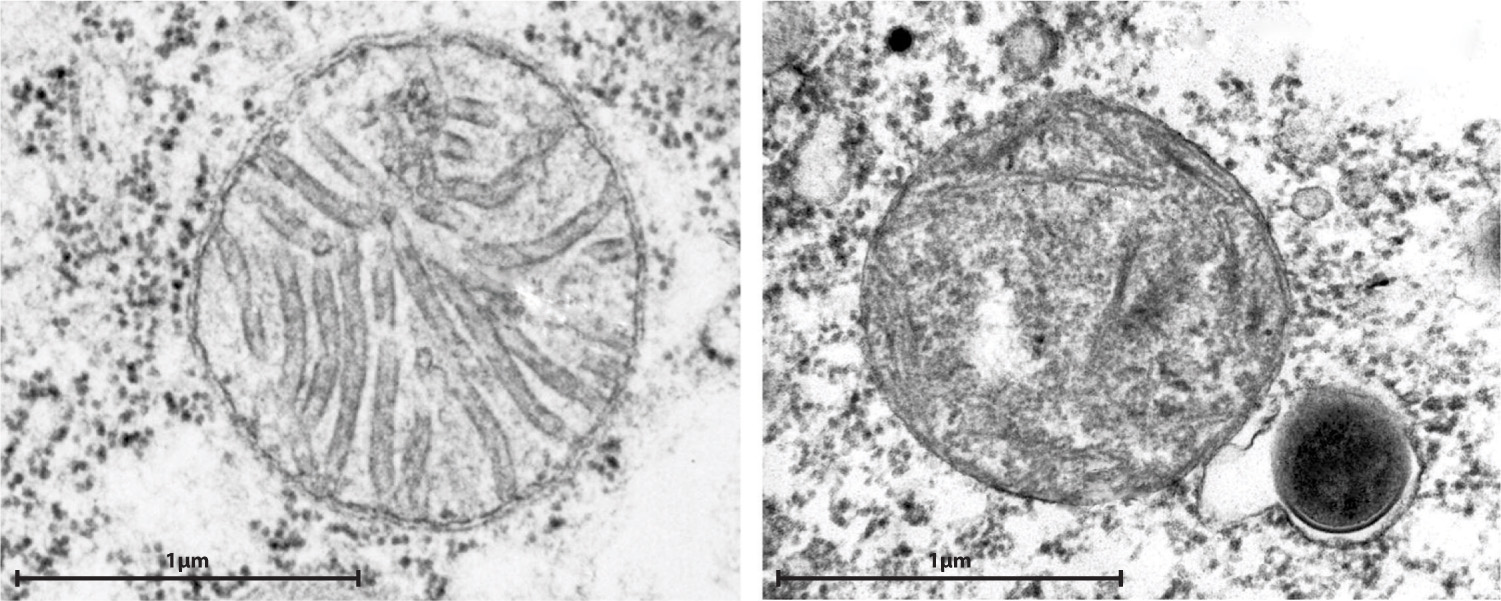
27-7
27.2.3 How did the eukaryotic cell originate?
Case 5 The Human Microbiome: Diversity Within
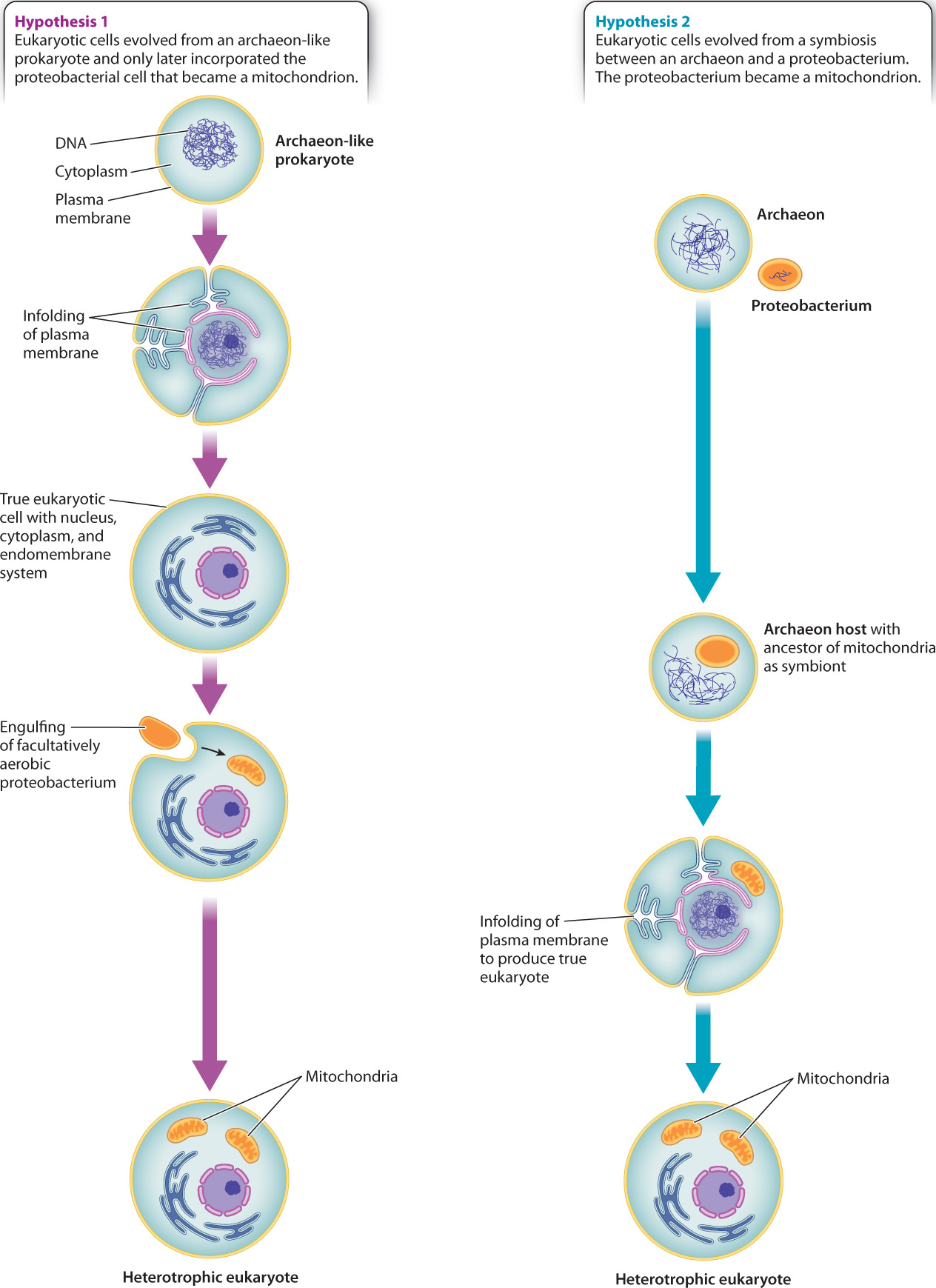
If mitochondria originated as proteobacteria and chloroplasts are descended from cyanobacteria, where does the rest of the eukaryotic cell come from? Analysis of the nuclear genome alone provides no clear picture because genes of bacterial, archaeal, and purely eukaryotic origin are all present. As discussed, many nuclear genes originated with the mitochondria and chloroplasts acquired from specific bacteria. However, genes from other groups of bacteria also reside in the eukaryotic nucleus, recording multiple episodes of horizontal gene transfer through evolutionary history. In contrast, some genes are present only in eukaryotes and apparently evolved after the domain originated. Still others are clearly related to the genes of Archaea, including the genes that govern DNA transcription and translation.
Two starkly different hypotheses have been proposed to explain this mix of genes. Some biologists believe that the host for mitochondrion-producing endosymbiosis was itself a true eukaryotic cell, with nucleus, cytoskeleton, and endomembrane system, but only limited ability to derive energy from organic molecules (Fig. 27.7). In this view, nuclear genes in Eukarya resemble those of Archaea because the primordial eukaryotic host cell was closely related to Archaea. Others, however, argue that no eukaryotic cell existed before there were mitochondria. Instead, they propose that the eukaryotic cell as a whole began as a symbiotic association between a proteobacterium and an archaeon (Fig. 27.7). The proteobacterium became the mitochondrion and provided many genes to the nuclear genome. The archaeon provided other genes, including those used to transcribe DNA and translate it into proteins.
Biologists continue to debate these alternatives. Both hypotheses address the hybrid nature of the eukaryotic genome, but neither explains the origins and evolution of the nucleus, linear chromosomes, the eukaryotic cytoskeleton, or a cytoplasm subdivided by ever-changing membranes. There is no consensus on the question of eukaryotic origins—it is one of biology’s deepest unanswered questions, awaiting novel observations by a new generation of biologists. Once a dynamic cytoskeleton became coupled to a flexible membrane system, however, the evolutionary possibilities of eukaryotic form were established.
27-8
27-9
27.2.4 In the oceans, many single-celled eukaryotes harbor symbiotic bacteria.
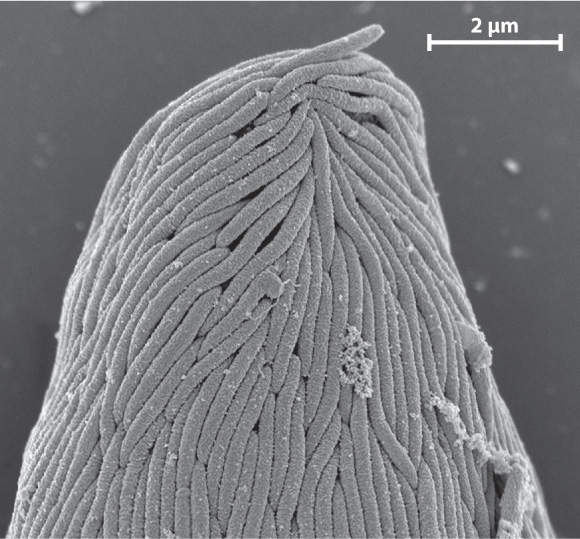
The evolution of the eukaryotic cell is one of intimate associations between formerly free-living organisms. An unexpected symbiosis was recently discovered in the Santa Barbara Basin, a local depression in the seafloor off the coast of southern California. Sediments accumulating in this basin contain little oxygen but large amounts of hydrogen sulfide (H2S) generated by anaerobic bacteria within the sediments. We might predict that eukaryotic cells would be uncommon in these sediments. Not only is oxygen scarce, but sulfide actively inhibits respiration in mitochondria. It came as a surprise, then, that samples of sediment from the Santa Barbara Basin contain large populations of single-celled eukaryotes.
Some of these cells have hydrogenosomes, mitochondria altered by evolution to generate energy where oxygen is absent. Others, however, appear to thrive by supporting populations of symbiotic bacteria on or within their cells. In one case, in which rod-shaped bacteria cover the surface of their eukaryotic host (Fig. 27.8), research has shown that the bacteria metabolize sulfide in the local environment, thereby protecting their host from this toxic substance. Scientists hypothesize that the bacteria benefit from this association as well because they get a free ride through the sediments, enabling them to maximize chemoautotrophic growth by remaining near the boundary between waters that contain oxygen and those rich in sulfide.
The diversity of these eukaryotic–bacterial symbioses is remarkable—and poorly studied. What has been learned to date, however, shows that single-celled eukaryotes have evolved numerous symbiotic relationships with chemoautotrophic bacteria, associations that feed and protect the eukaryotes, enabling them to colonize habitats where most eukaryotes cannot live. Clearly, then, the types of symbioses that led to mitochondria and chloroplasts on the early Earth were not rare events, but basic associations between cells that continue to evolve today.