27.3 EUKARYOTIC DIVERSITY
Historically, Domain Eukarya was divided into four kingdoms: plants, animals, fungi, and protists. Plants, animals, and fungi received special consideration for the obvious reason that they include large and relatively easily studied species. All remaining eukaryotes were grouped together as protists, which were defined as organisms having a nucleus but lacking other features specific to plants, animals, or fungi. The term “protist” is frowned on by some biologists because it doesn’t refer to a monophyletic grouping of species—that is, an ancestral form and all its descendants. Nonetheless, as an informal term that draws attention to a group of organisms sharing particular characteristics, “protist” usefully describes the diverse world of microscopic eukaryotes and seaweeds.
Two other terms scorned by systematists but embraced by ecologists are “algae” and “protozoa.” Algae are photosynthetic protists. They may be microscopic single-celled organisms or the highly visible, multicelled organisms we call seaweed. Protozoa are heterotrophic protists. These are almost exclusively single-celled organisms. “Algae” and “protozoa” in fact are simple and useful terms that have little phylogenetic meaning but which convey a great deal of information about the structure and function of these organisms.
Protist cells exhibit remarkable diversity. Some have cell walls, while others do not. Some make skeletons of silica (SiO2) or calcium carbonate (CaCO3), and others are naked or live within tests, or “houses,” constructed exclusively of organic molecules. Some are photosynthetic, while others are heterotrophic. Some move by beating flagella, and others, like Amoeba, can extend fingers of cytoplasm, called pseudopodia, to move and capture food. Most are aerobic, but there are anaerobic protists as well. Surprisingly, most of these distinctive features have evolved multiple times and so do not define phylogenetically coherent groups. As a result, our understanding of evolutionary pattern in Eukarya had to wait for the molecular age.
27-10
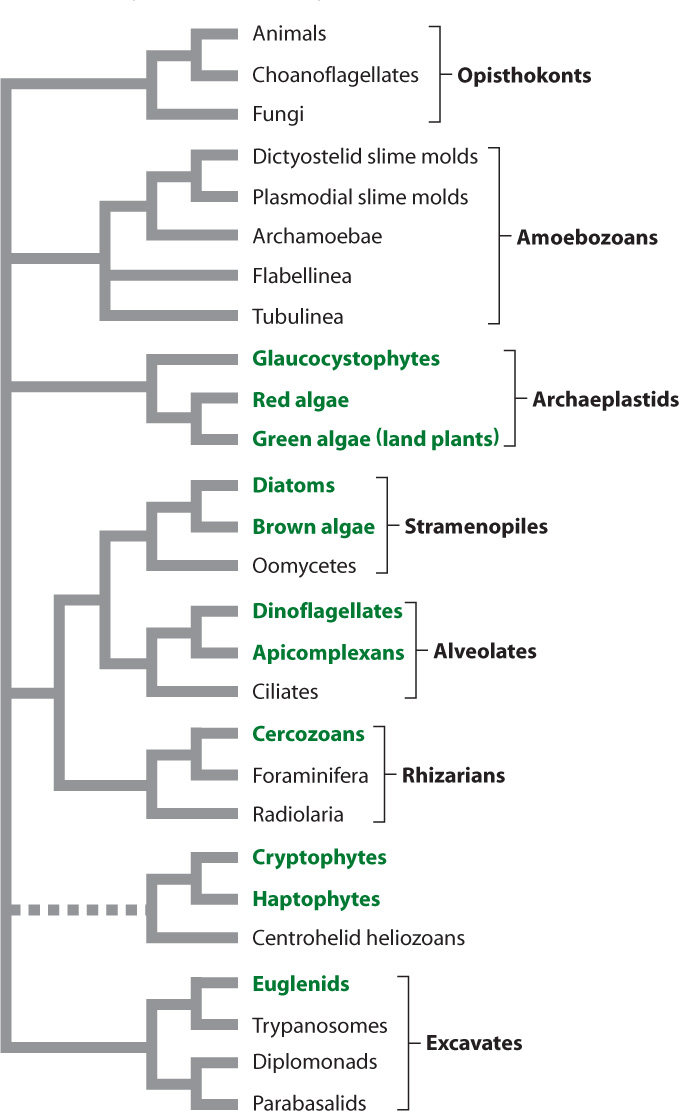
Fig. 27.9 shows a phylogenetic tree of eukaryotes as biologists currently understand it. As in the case of Bacteria, it is easier to identify major groups of Eukarya than to establish their evolutionary relationships to one another. Molecular sequence comparisons consistently recognize seven major groups, commonly called superkingdoms, noted in the figure. Animals fall within one of these superkingdoms, the Opisthokonta, whereas plants fall within another, the Archaeplastida. Most, but not all, of the divisions into these major eukaryotic branches are strongly supported by data from genes and genomes. A few groups, notably the cryptophytes, haptophytes, and centrohelid heliozoans, seem to wander from position to position from one phylogenetic analysis to the next, sometimes moving together, sometimes separately. Whether the well-resolved superkingdoms constitute the only major limbs on the eukaryotic tree is less certain. Many microscopic protists remain unstudied, and we may yet be surprised by what they ultimately show.
In the following sections, we introduce the major features of eukaryotic diversity. The species numbers we present indicate known taxonomic diversity, and we emphasize that most protistan species remain unknown. Many biologists estimate that only about 1 in 10 protist species has been described so far. As this is also the case for animals and fungi (but arguably not for land plants because they are all visible to the naked eye), the relative numbers of species within different superkingdoms may reflect something like the actual distribution of biodiversity among eukaryotes.
27.3.1 Our own group, the opisthokonts, is the most diverse eukaryotic superkingdom.
Over the past 250 years, biologists have described about 1.8 million species. Of these, 75% or so fall within the Opisthokonta (Fig. 27.10), a name derived from Greek words meaning “posterior pole,” calling attention to the fact that cell movement within this group is propelled by a single flagellum attached to the posterior end of the cell. Not all cells in this superkingdom move around. In humans, for example, the flagellum is limited mostly to sperm. Opisthokonts are heterotrophic, although some species harbor photosynthetic symbionts. Animals are the most diverse and conspicuous opisthokonts. More than 1.3 million animal species, mostly insects and their relatives, have been described. Fungi are also diverse, with more than 75,000 described species, including the visually arresting mushrooms (Fig. 27.10a). Here, however, we focus on opisthokont protists, somewhat poorly known microorganisms that hold clues to the origins of complex multicellularity in this superkingdom (Chapter 28).
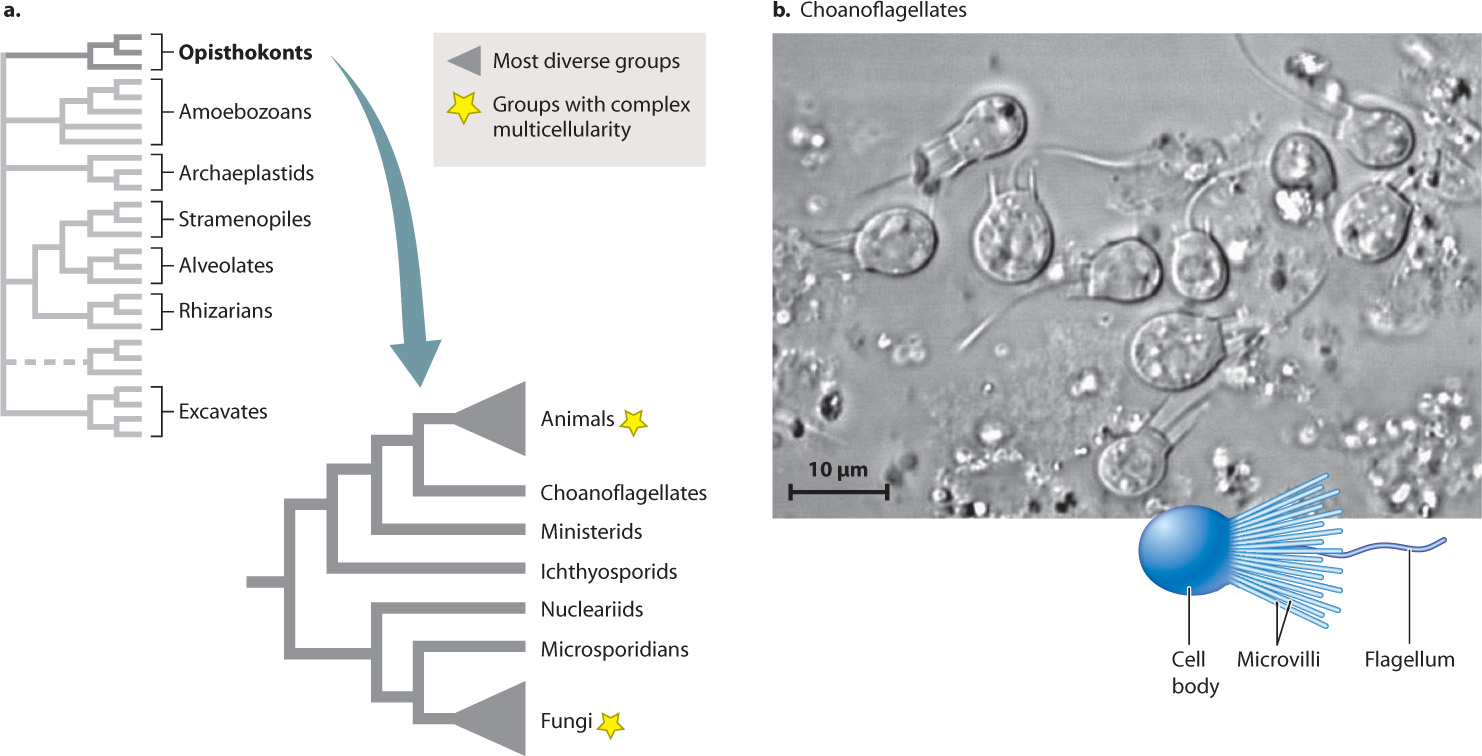
It isn’t easy to find morphological characters that unite all opisthokonts. We face this difficulty partly because animals and fungi have diverged so strikingly from what must have been the ancestral condition of the group. However, as molecular sequence comparisons began to reshape our understanding of eukaryotic phylogeny, it soon became clear that fungi and animals are closely related.
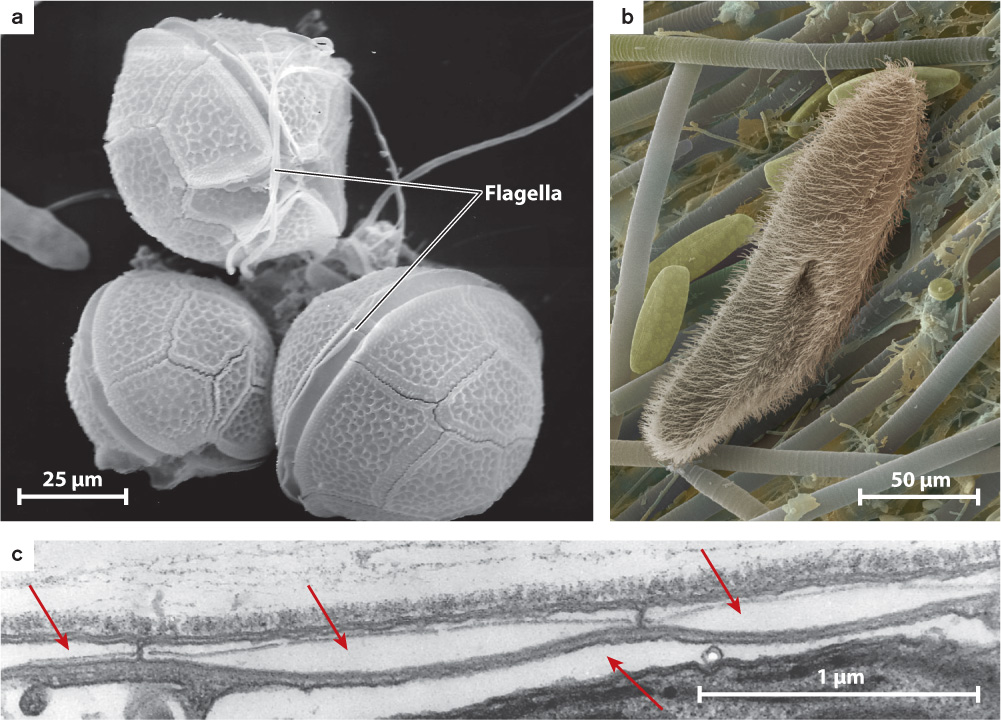
Even more closely related to the animals are choanoflagellates, a group of mostly unicellular protists characterized by a ring of microvilli, fingerlike projections that form a collar around the cell’s single flagellum (Fig. 27.10b). About 150 choanoflagellate species have been described from marine and freshwater environments, where they prey on bacteria. As early as 1841, the close similarity between choanoflagellates and the collared feeding cells of sponges suggested that these minute organisms might be our closest protistan relatives. This view gained widespread popularity with the discovery in 1880 of Proterospongia haeckeli, a choanoflagellate that lives in colonies of cells joined by adhesive proteins.
27-11
Molecular sequence comparisons now confirm the close relationship between animals and choanoflagellates. For example, the complete genome of the choanoflagellate Monosiga brevocollis shows that a number of signaling molecules known to play a role in animal development are present in our choanoflagellate relatives, although their function in these single-celled organisms remains largely unknown. More generally, many genes once thought to be unique features of animals have now been identified in choanoflagellates, underscoring the deep evolutionary roots of animal biology.
Microsporidia form another group of single-celled opisthokonts. Microsporidia are parasites that live inside animal cells. Only their spores survive in the external environment, where they await the opportunity to infect a host and complete their life cycle. Microsporidia infect all animal phyla, so the approximately 1000 known species probably represent only the tip of the iceberg. More than a dozen species have been isolated from human intestinal tissues. Microsporidia infections can be particularly devastating in AIDS patients. Beyond their importance in human health, microsporidians have attracted the attention of biologists because of features they lack: Microsporidian cells have no aerobically respiring mitochondria, no Golgi apparatus, and no flagella. Furthermore, they have a highly reduced metabolism and among the smallest genomes of any known eukaryote. The cellular simplicity of microsporidians does not mean that they are early-evolved organisms, however. Molecular sequencing studies show that microsporidians are the descendants of more complex organisms. Their simplicity then is an adaptation for life as an intracellular parasite. It is now widely accepted that microsporidians are closely related to the fungi.
Other protistan opisthokonts have been identified in recent years. These are mostly parasites of aquatic animals, but like the choanoflagellates described earlier, their biology is beginning to illuminate the evolutionary path to complex multicellularity in animals and fungi.
27-12
27.3.2 Amoebozoans include slime molds that produce multicellular structures.
As their name implies, the superkingdom Amoebozoa (Fig. 27.11) is a group of eukaryotes with amoeba-like cells that move and gather food by means of pseudopodia (illustrated by Amoeba proteus in Fig. 27.11b). More than 1000 amoebozoan species have been described. In some amoebozoan species, large numbers of cells aggregate to form multicellular structures as part of their life cycle.
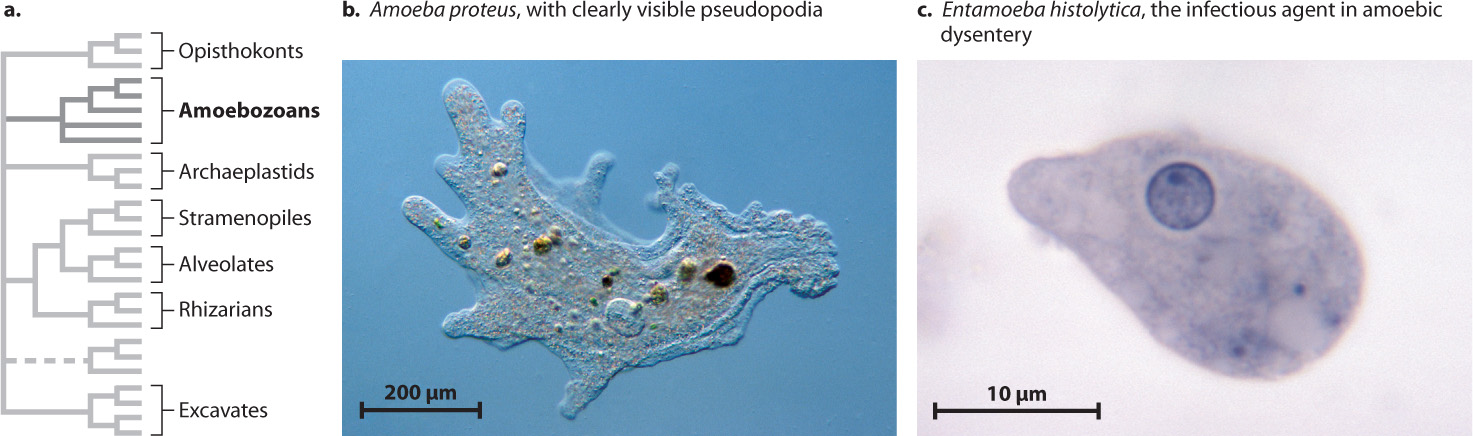
Although there are amoeba-like cells that are not members of the Amoebozoa superkingdom, all members of this group are basically amoeboid in cellular organization, at least at some stage of their life cycle. Amoebozoans play an important role in soils as predators on other microorganisms. Some amoebozoans also influence human health. Entamoeba histolytica (Fig. 27.11c), an anaerobic protist that causes amoebic dysentery, is responsible for 50,000–100,000 deaths every year.
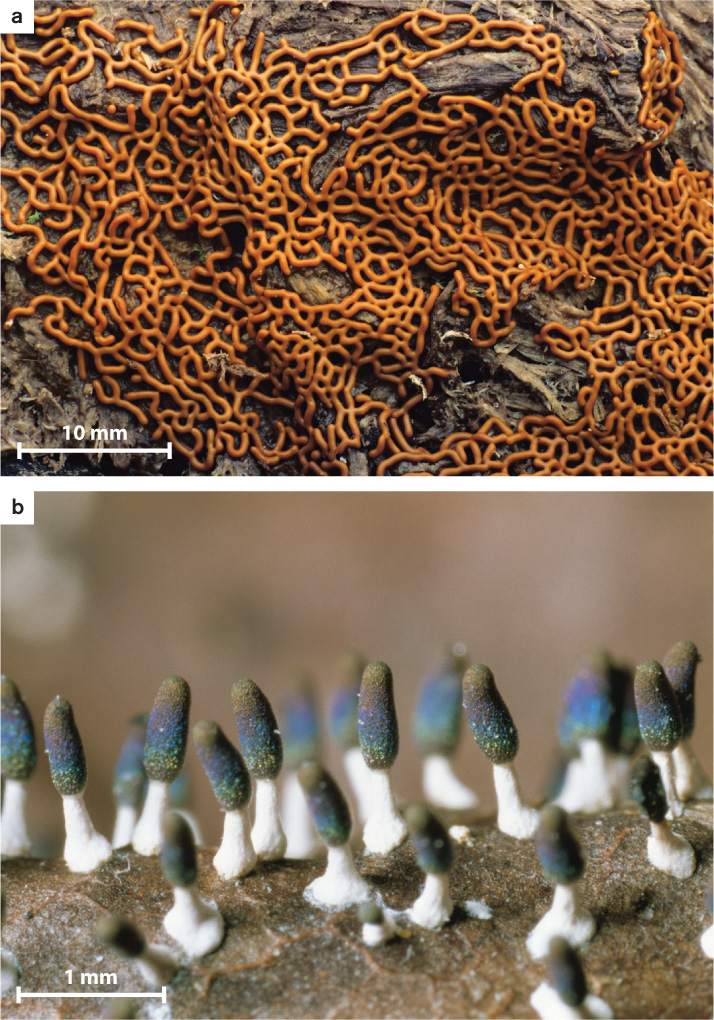
Beyond considerations of human health, the amoebozoans of greatest biological interest are the slime molds. In plasmodial slime molds (Fig. 27.12), haploid cells fuse to form zygotes that subsequently undergo repeated rounds of mitosis but not cell division to form colorful, often lacy structures visible to the naked eye (Fig. 27.12a). These structures, called plasmodia, are coenocytic, which means they contain many nuclei within one giant cell. The plasmodia can move and so seek out and feed on the bacteria and small fungi commonly found on bark or plant litter on the forest floor. Triggered by poorly understood environmental signals, plasmodia eventually differentiate to form stalked structures called sporangia that can be 1 to 2 mm high (Fig. 27.12b). Within the mature sporangium, cell walls form around the many nuclei, producing discrete cells that undergo meiosis, generating haploid spores that disperse into the environment. Germination of these spores begins the life cycle anew.
Cellular slime molds (Fig. 27.13) spend most of their life cycle as solitary amoeboid cells feeding on bacteria in the soil. Starvation, however, causes cells to produce the chemical signal cyclic AMP (Chapter 9), which induces as many as 100,000 cells to aggregate into a large multicellular slug-like form (Fig. 27.13a). This “slug” can migrate by a coordinated movement of its cells governed by actin and myosin, the same proteins that control muscle movement in animals. Migrating “slugs” can forage for food, leaving behind a trail of mucilage as they move—that’s why they’re called slime molds. Like plasmodial slime molds, the “slugs” of cellular slime molds eventually differentiate to form stalk-borne sporangia that produce the spores responsible for dispersal of the organism and renewal of the life cycle (Fig. 27.13b).
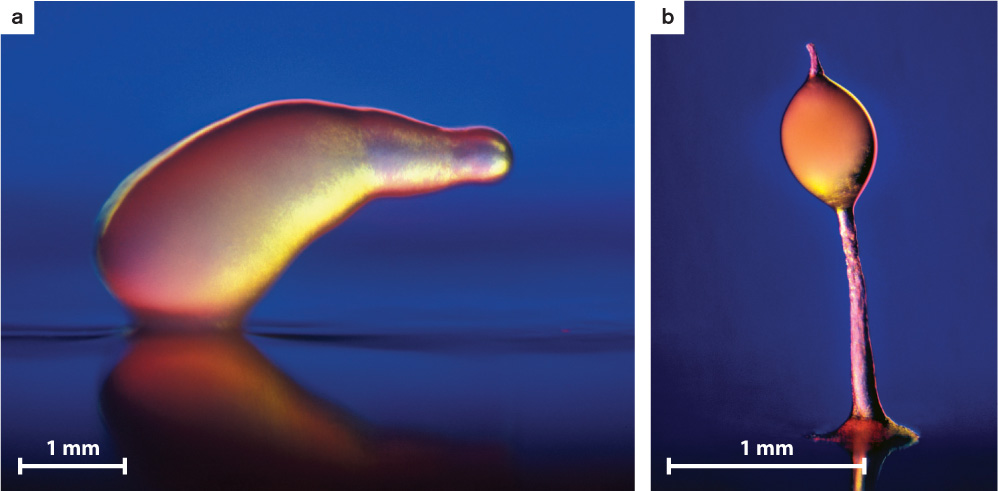
27-13
Slime molds are not true intermediates in the evolution of multicellularity—that is, they are not closely related to animals, fungi, or other groups in which complex multicellularity evolved. Nonetheless, the cellular slime mold Dictyostelium has been extensively studied by biologists as a model organism because it has much to teach us about fundamental eukaryotic processes of cell signaling and differentiation, cellular motility, and mitosis.
27.3.3 Archaeplastids are photosynthetic organisms, including land plants.
Next to the opisthokonts, the most conspicuous and diverse eukaryotes fall within the superkingdom Archaeplastida, another major branch of the eukaryotic tree (Fig. 27.14a). This is where we find the land plants, whose 300,000 described species dominate eukaryotic biomass on this planet (Chapter 33). The archaeplastids contain three major phylogenetic groups, and with the exception of a few parasitic and saprophytic (living on dead matter) species, all are photosynthetic. This indicates that the last common ancestor of living archaeplastids was photosynthetic. In fact, archaeplastids are the direct descendants of the protist that first evolved chloroplasts from endosymbiotic cyanobacteria. Some molecular analyses also place the group that includes cryptophyte and haptophyte algae within this branch, but this remains a topic of continuing research.
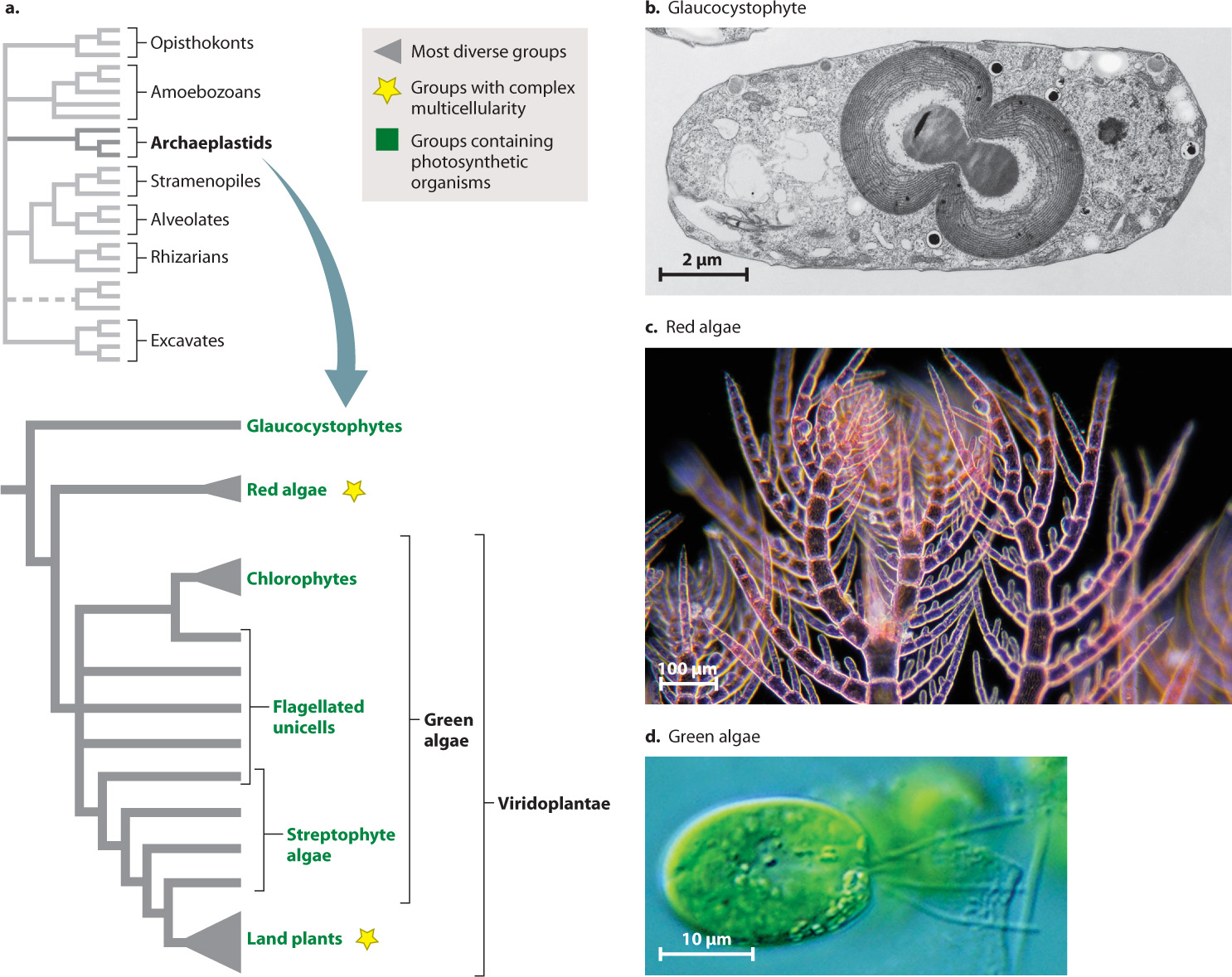
The least conspicuous group of archaeplastids are the glaucocystophytes (Fig. 27.14b), a small group of single-celled algae found in freshwater ponds and lakes. We might skip over them entirely, except for one illuminating observation: Glaucocystophyte chloroplasts appear to retain more features of the ancestral cyanobacterial endosymbiont than any other algae. Their chloroplasts have walls of peptidoglycan, the same molecule found in cyanobacterial walls, and their photosynthetic pigments include biliproteins, also found in cyanobacteria.
More abundant and diverse is the second major archaeplastid group, the red algae (Fig. 27.14c). About 5000 species are known, mostly from marine environments. Most are multicellular and some have differentiated tissues and complex morphology. Like other archaeplastids, most also have walls made of cellulose. The principal photosynthetic pigments in red algae are a form of chlorophyll called chlorophyll a and the biliproteins also found in glaucocystophytes and most cyanobacteria.
Red algae can be conspicuous as seaweeds on the shallow seafloor. One subgroup of red algae, known as the coralline algae, secretes skeletons of calcium carbonate (CaCO3) that deter predators and help the algae withstand the energy of waves in shallow marine environments. If you examine the margin of coral reefs, where waves crash into the reef, you will often see that it is coralline algae and not the corals themselves that resist breaking waves and so enable reefs to expand upward into wave-swept environments. While most common and diverse in near-shore environments, red algae can also grow where light penetrates most deeply in the ocean. Their photosynthetic pigments enable them to take advantage of the short (blue) wavelengths of light.
Red algae are important as food in certain cultures, for example, laver in the British Isles (particularly in Wales, in cakes similar to oatcakes) and nori in Japan. Without knowing it, you often ingest carageenan, a polysaccharide synthesized by red algae. Carageenan molecules stabilize mixtures of biomolecules and promote gel formation, and so they are used in products ranging from ice cream to toothpaste. Agar, another red algal polysaccharide, is found universally in microbiology laboratories because the gel it forms at room temperature provides a nearly ideal substrate for bacterial growth.
27-14
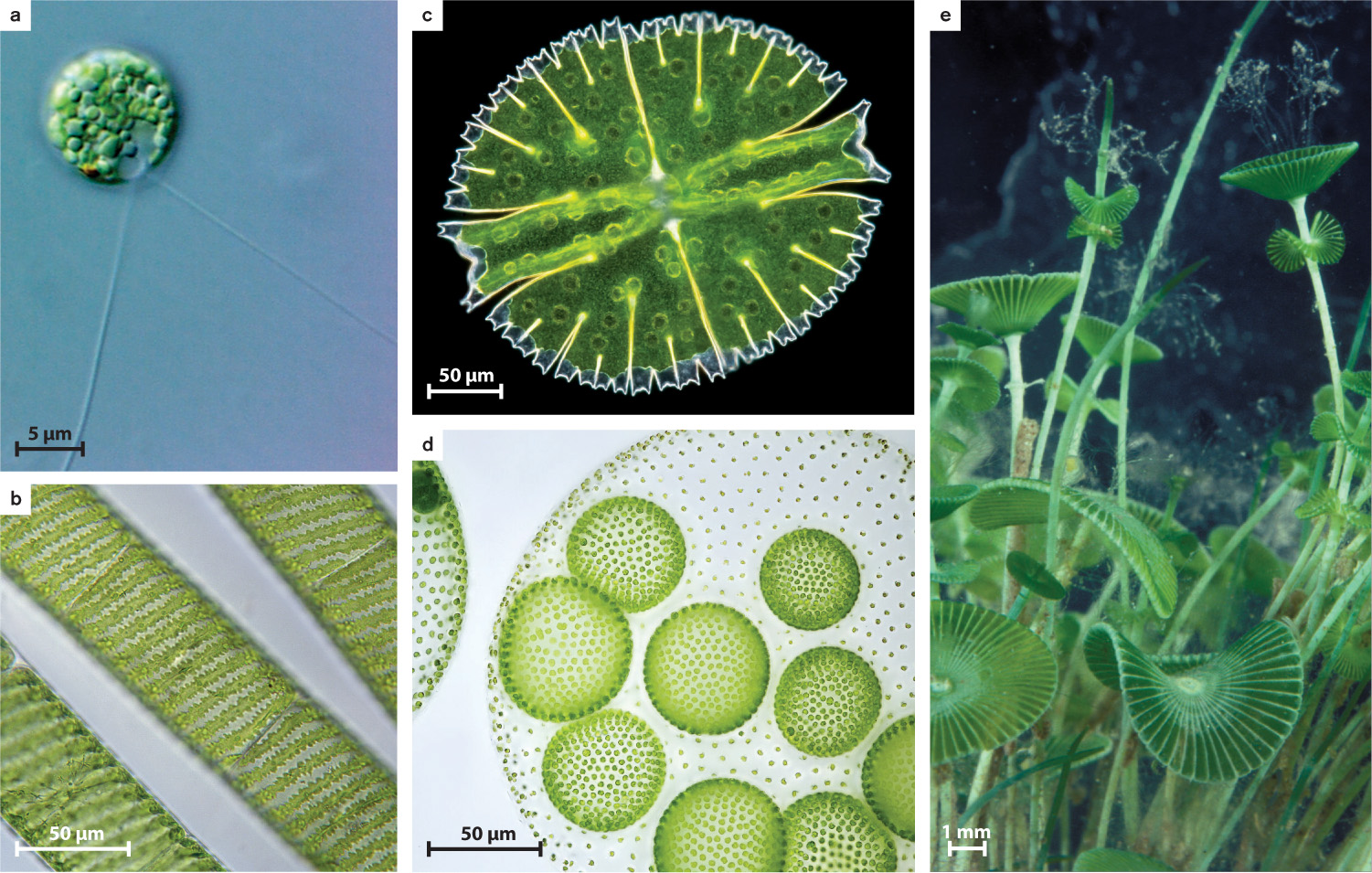
The land plants and their algal relatives form Viridoplantae, the third branch of Archaeplastida. In this chapter, we are concerned with the protistan members of this group, the green algae (Fig. 27.14d). Green algae include a diverse assortment of species (about 10,000 have been described) that live in marine and, especially, freshwater environments. Green algae range from tiny single-celled flagellates to meter-scale seaweeds (Fig. 27.15). They all are united by two features: the presence of chlorophyll a and chlorophyll b in chloroplasts that have two membranes, and a unique attachment for flagella.
It is thought that green algae originated as small flagellated cells in seawater, and today most species that branch early on the green algal tree live as photosynthetic cells in the water. These species are called phytoplankton, and one of them is illustrated in Fig. 27.15a. The larger diversity of green algae, however, is found on two branches that diverged from these flagellated ancestors. One branch, the chlorophytes, radiated mostly in the sea and includes common seaweeds such as sea lettuce, which is seen globally on seashores as flotsam. The chlorophyte branch also includes relatively large and complex seaweeds that can form structures up to tens of centimeters long in tropical and temperate oceans (Fig. 27.15e). Moreover, chlorophyte green algae have played a major role in laboratory studies of photosynthesis (Chlamydomonas, Fig. 27.15a) and multicellularity (Volvox, Fig. 27.15d).
27-15
For humans, however, the more important branch of the green algal tree is the one that diversified on land. Called streptophytes, the species on this branch show a progression of form from unicells at the base through simple cell clusters and filaments on intermediate branches (see Figs. 27.15b and 21.15c), to complex multicellular algae that form the closest relatives of land plants (Chapter 33). As was true of choanoflagellates and animals, green algae display features of cell biology and genomics that tie them unequivocally to land plants.
Question Quick Check 2
TQBUO7GeKNWYaUFTo7Bfq/MsyCu3403cuKYW+IyN3+RUIXdF8MMm2z+BBmp5SvwpsZXyvvNjmu1/cqsXwtHNew==27.3.4 Other photosynthetic organisms occur in the stramenopiles and alveolates.
The superkingdom Stramenopila forms a diverse and distinctive branch of the eukaryotic tree (see Fig. 27.9). The group includes unicellular organisms and giant kelps, algae and protozoa, free-living cells and parasites. All have an unusual flagellum that bears two rows of stiff hairs (Fig. 27.16). Most also have a second, smooth flagellum.
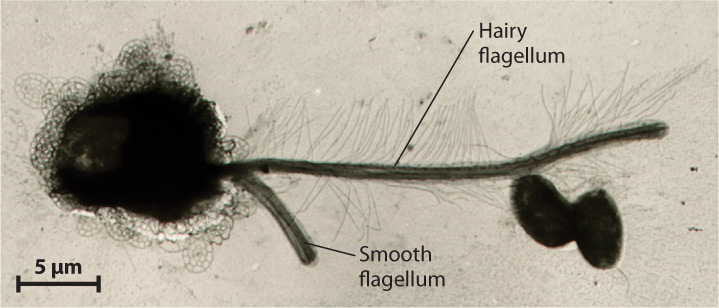
27-16

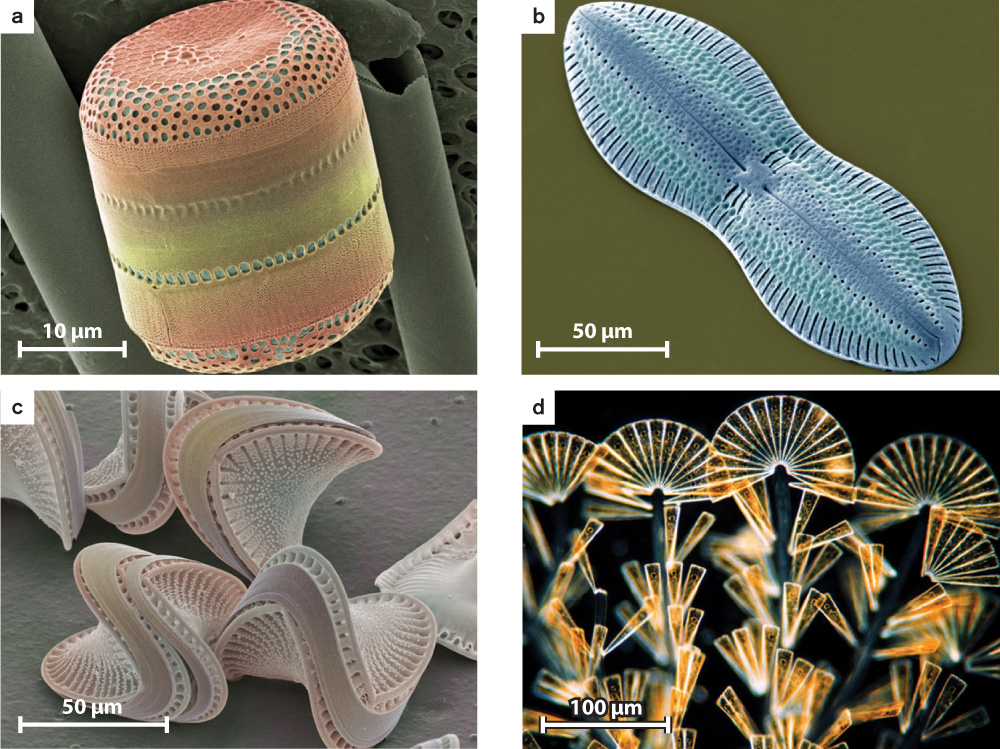
Despite the presence of heterotrophic species, most stramenopiles are photosynthetic. There are about a dozen groups of stramenopile algae, of which the diatoms and the brown algae deserve special mention. Brown seaweeds are common along rocky shorelines across the world, and in the Sargasso Sea, huge masses of ropy brown algae (aptly named Sargassum) float at the surface. Easily the most impressive brown algae are the kelps, giant seaweeds that form forests above the seafloor (Fig. 27.17). Kelps not only develop functionally distinct tissues, but have evolved distinct organs specialized for attachment, photosynthesis, flotation, and reproduction.
The most diverse stramenopiles, however, are the diatoms (Fig. 27.18). Recognized by their distinctive skeletons of silica, diatoms account for half of all primary production in the sea (and, so, nearly a quarter of all photosynthesis on Earth). The 10,000 known diatom species thrive in environments that range from wet soil to the open ocean.
Among heterotrophic stramenopiles, the oomycetes, or water molds, are particularly interesting. Originally classified as fungi, these multicellular pathogens cause a number of plant diseases, including the potato blight that devastated Ireland during the 1840s (Chapter 33). Other species infect animals, including humans.
Like stramenopiles, the superkingdom Alveolata is a diverse group. It includes dinoflagellates (Fig. 27.19a), less than half of which contain chloroplasts and are photosynthetic, and ciliates (Fig. 27.19b), heterotrophic protists that have two nuclei in each cell and numerous short flagella called cilia. The group is united by the presence of cortical alveoli, small vesicles packed beneath the cell surface that, in some species at least, store calcium ions for use by the cell (Fig. 27.19c). Dinoflagellates are important as photosynthesizers in many parts of the oceans, and they affect humans when they form red tides, large and toxic blooms in coastal waters. Red tides occur when nutrients are supplied to coastal waters in large amounts. This may occur for natural reasons, but it commonly reflects human activities such as sewage seeps or runoff from highly fertilized croplands.
Scientists have described nearly 4000 dinoflagellate and 8000 ciliate species, but it may be the third principal group of alveolates, the Apicomplexans, that attracts most scientific attention. The reason is simple: The apicomplexan Plasmodium falciparum and related species cause malaria, a lethal disease that sickened millions and killed more than 600,000 people in 2011 (Case 4: Malaria). Although Plasmodium cells are heterotrophic, they contain vestigial chloroplasts that suggest evolutionary loss of photosynthesis. Recently, this hypothesis has been confirmed by the discovery in Sydney Harbour, Australia, of photosynthetic cells closely related to parasitic Plasmodium.
27.3.5 Photosynthesis spread through eukaryotes by repeated endosymbioses involving eukaryotic algae.
A curious feature of the eukaryotic tree is that photosynthetic eukaryotes are distributed widely but discontinuously among its branches (see Fig. 27.9). How can we explain this pattern?
Two hypotheses have been suggested: Either photosynthesis was established early in eukaryotic evolution and was subsequently lost in some lineages, or eukaryotes acquired photosynthesis multiple times by repeated episodes of endosymbiosis. The second hypothesis, it turns out, is correct. The twist is that most of the symbionts involved in the spread of photosynthesis across eukaryotic phylogeny were photosynthetic eukaryotes, not cyanobacteria.
Once more, DNA sequence comparisons provide the decisive data. We can construct phylogenetic trees for photosynthetic eukaryotes using chloroplast genes and then repeat the exercise using nuclear genes. Fig. 27.20a shows a phylogenetic tree for chloroplasts. Notice that this tree differs substantially from the branching order in trees based on comparison of nucleotide sequences for nuclear genes, such as the tree shown in Fig. 27.20b. The differences between the chloroplast and nuclear trees favor the hypothesis that photosynthesis spread through the eukaryotes by means of repeated symbiotic events.
Fig. 27.20: How did photosynthesis spread through the Eukarya?
BACKGROUND Many different branches on the eukaryotic tree include photosynthetic species, commonly interspersed with non-photosynthetic lineages. Did eukaryotes gain photosynthesis once, by the endosymbiotic incorporation of a cyanobacterium, and then lose this capacity multiple times? Or, is the history of photosynthetic endosymbioses more complicated, involving multiple events of symbiont capture and transformation?
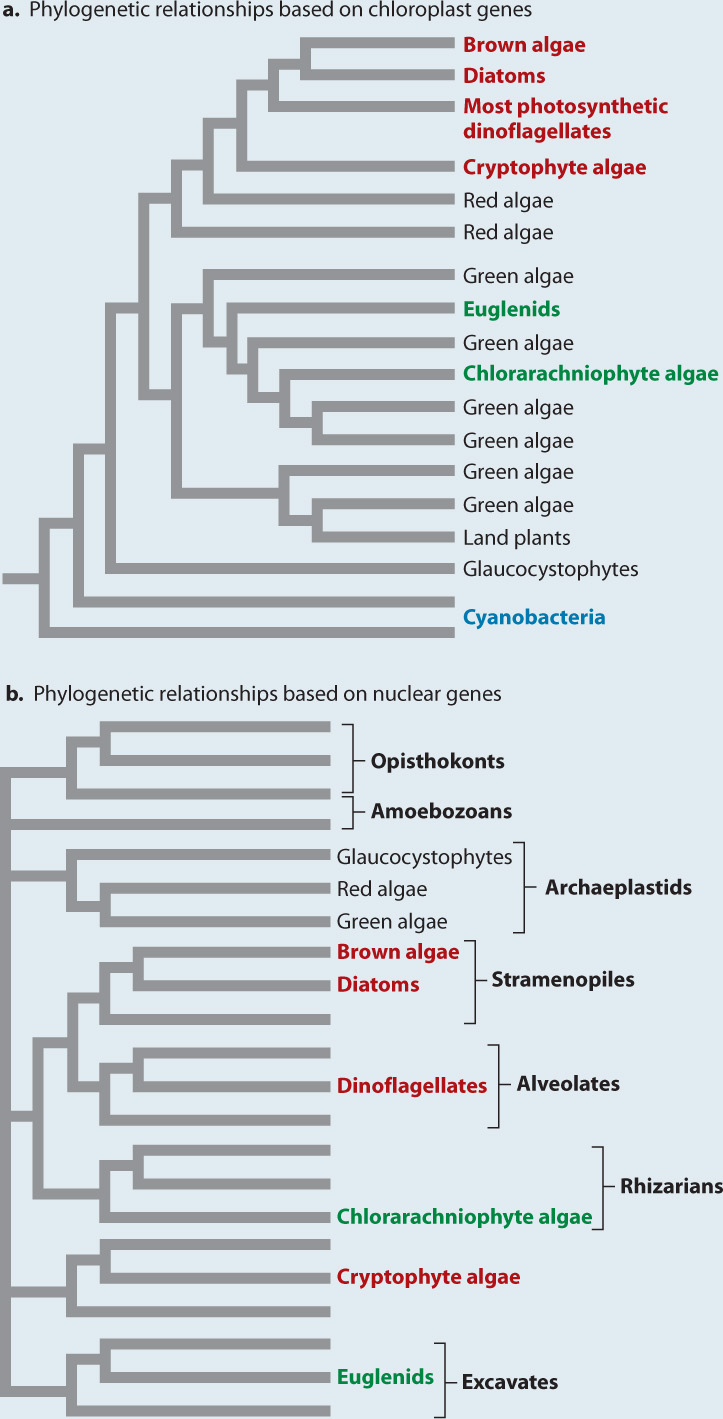
HYPOTHESIS 1 Photosynthesis was established early in eukaryotic evolution and was subsequently lost in some lineages.
HYPOTHESIS 2 Eukaryotes acquired photosynthesis multiple times by repeated episodes of endosymbiosis.
PREDICTIONS The two hypotheses make different predictions. If photosynthesis were acquired in a single common ancestor of all living eukaryotes and then lost in some lineages, we would expect chloroplast and nuclear gene phylogenies to show the same pattern of branching. If photosynthesis were acquired multiple independent times, we would expect different chloroplast and nuclear phylogenies to show different patterns.
EXPERIMENT Chloroplasts have DNA, so scientists developed a phylogenetic hypothesis based on molecular sequence comparison of chloroplast genes (Fig. 27.20a). This was then compared with a second phylogeny, for all eukaryotes, based on molecular sequence comparison of nuclear genes (Fig. 27.20b).
RESULTS Molecular sequence comparisons show that evolutionary relationships among chloroplasts do not mirror those based on nuclear genes. The chlorarachniophyte algae and photosynthetic euglenids (shown in green) fall in a monophyletic group with the green algae when chloroplast DNA is analyzed (Fig. 27.20a). However, these groups lie far from green algae in the phylogeny based on nuclear genes (Fig. 27.20b). Similarly, brown algae, diatoms, most photosynthetic dinoflagellates, and cryptophyte algae (shown in red) form a monophyletic group with the red algae in the phylogeny based on chloroplast DNA (Fig. 27.20a), but analysis of nuclear genes places them in different groups (Fig. 27.20b).
CONCLUSION The chloroplast and nuclear phylogenies show different patterns of branching, supporting the hypothesis that photosynthesis spread through the Eukarya by means of multiple eukaryotic endosymbionts.
SOURCE Hackett, J. D., H.S. Yoon, N. J. Butterfield, M. J. Sanderson, and D. Bhattacharya. 2007. “Plastid Endosymbiosis: Sources and Timing of the Major Events.” In Evolution of Primary Producers in the Sea, edited by P. G. Falkowski and A. H. Knoll, 109–132. Boston: Elsevier Academic Press.
27-17
Let’s look more closely at the chloroplast phylogeny in Fig. 27.20a. The lower part of the phylogeny shows that chloroplasts form a monophyletic grouping nested within the cyanobacteria. This is a principal line of evidence supporting the endosymbiotic origin of chloroplasts. Moving upward into the chloroplast branch, if we look only at the branches shown in black, we see the phylogeny indicated by nuclear genes for the archaeplastids—glaucocystophytes are sister to red algae and green algae. But notice the branches in green that sprout from within the green algae, the chlorarachniophytes and the euglenids. Both of these groups have chloroplasts that contain the same pigments as green algae (chlorophyll a and chlorophyll b), and molecular sequence comparison clearly indicates that their chloroplasts are closely related to the chloroplasts of green algae. Nuclear genes, however, place these groups far from the archaeplastids. Chlorarachniophytes are found within the superkingdom Rhizaria, and euglenids fall among the superkingdom Excavata. How do we explain this discordance? The answer is that chlorarachniophytes gained photosynthesis by establishing green algal cells (containing chloroplasts derived from an earlier symbiotic assimilation of cyanobacteria) as endosymbionts. Euglenids did the same thing, independently.
27-18
27-19
Now focus on the branch dominated by red algae. Once again, the branches shown in red indicate photosynthetic eukaryotes whose nuclear genes place them far away from red algae on phylogenetic trees. Thus, groups like the photosynthetic stramenopiles (diatoms, kelps, and others), photosynthetic dinoflagaellates, and a few others also appear to have arisen by endosymbiosis involving another photosynthetic endosymbiont that was eukaryotic—in this case, a red algal cell.
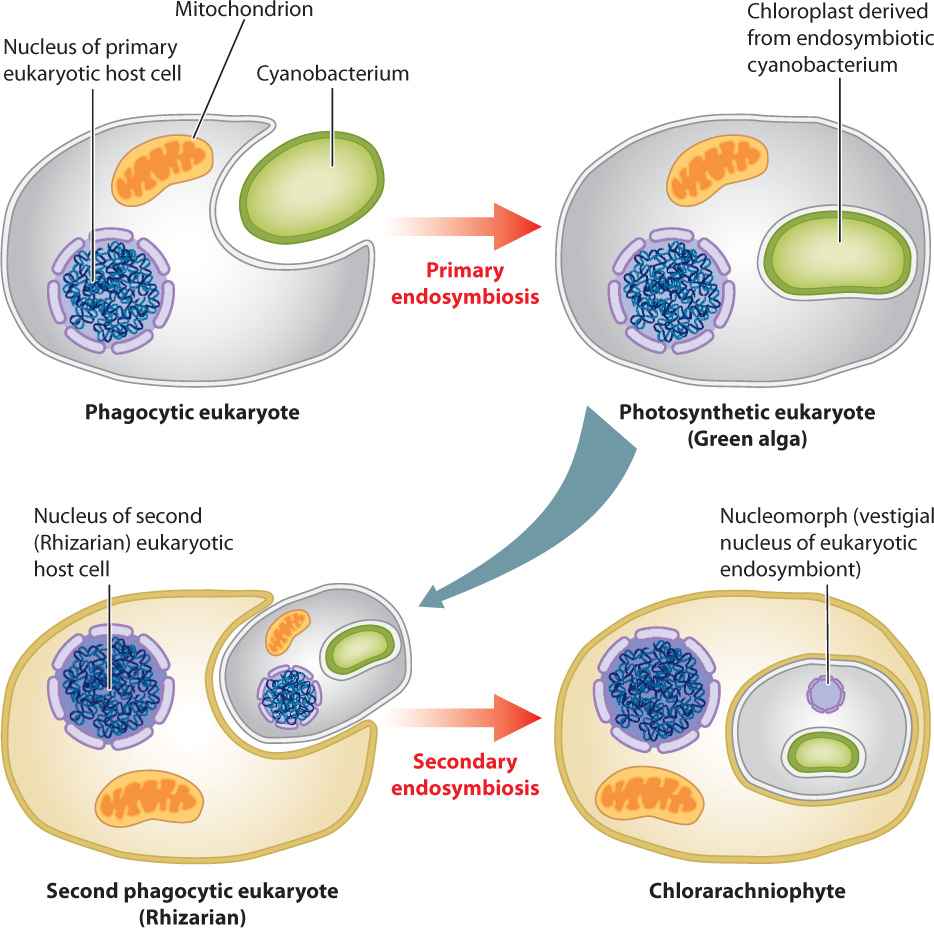
Several features of cell biology support this view. We noted earlier that chloroplasts in green and red algae are bounded by two membranes, but most of the algae shown in red and green in Fig. 27.20 have four membranes around their chloroplasts. The presence of four membranes is expected under the hypothesis of eukaryotic endosymbionts: The inner two membranes are those that bounded the chloroplast in the green or red algal endosymbiont, the third membrane is derived from the outer cell membrane of the symbiont, and the fourth, outer membrane is part of the cell membrane of the host (Fig. 27.21).
Chlorarachniophyte (and, on the red branch, cryptophyte) algae also retain another feature that supports the eukaryotic origin of their chloroplasts: a small organelle called a nucleomorph. The nucleomorph in chlorarachniophyte algae is, in fact, the remnant nucleus of a green algal symbiont. It retains only a few genes, but those show close phylogenetic affinity with green algae. Nucleomorph genes in cryptophyte algae similarly provide evidence for a red algal symbiont.
How many endosymbiotic events are required to account for the diversity of photosynthetic eukaryotes? There was the primary endosymbiosis that gave rise to green and red algae, two further events that established chloroplasts derived from green algae in euglenids and chlorarachniophytes, and at least one to three events that established chloroplasts derived from red algae in cryptophytes, haptophytes, and stramenopiles.
The dinoflagellates add a remarkable coda to this accounting. Like stramenopiles, most dinoflagellates have chloroplasts derived from red algae. Three dinoflagellate groups, however, have chloroplasts that are clearly derived from green, cryptophyte, and haptophyte algae. Include Paulinella and at least 8 to 10 endosymbiotic events are required to explain eukaryotic photosynthesis. Eukaryotes never evolved oxygenic photosynthesis—only the cyanobacteria accomplished that. Instead, eukaryotes appropriated the biochemistry of cyanobacteria and spread it throughout the domain by the horizontal transfer of entire cells.
Question Quick Check 3
HCbAMvL+G5qntI5xKac8UYcV3LkKCv3whDh/DbFnTovNE7Th7TNFP6SWRPx88LFUex+43cw8HeXMppWOVD3NfKVXlKnMt1Kz3B+8mR6p5FFOogGbABlzi53qjR/F7nSeJlbiyFEUhTAnwZX9uvKdrAXAVufepuW6IRGN/Q==27.3.6 The first branch of the eukaryotic tree may separate animals and slime molds from plants and diatoms.
Molecular sequence comparisons have succeeded in dividing eukaryotic species into the seven superkingdoms, but determining relationships among these major branches has proved difficult. This uncertainty is apparent in Fig. 27.9, which shows seven major branches of eukaryotic organisms but, for the most part, does not suggest how they relate to one another. Biologists call this an unrooted tree because we cannot go from the tips of the finest branches (present-day organisms) and trace backward through successive branch points to arrive at the “root”—the last common ancestor of the total group.
27-20
As discussed in Chapter 23, biologists use synapomorphies, features shared by some but not all members of a group through descent from a common ancestor, to recognize sister groups, or closest evolutionary relatives. Skull shape, for example, unites birds and crocodiles, while separating them from other tetrapod vertebrates. Can we find characters that will allow us to identify sister-group relationships among the eukaryotic superkingdoms? It isn’t easy, because the groups diverged more than a billion years ago and have accumulated so many differences in their cellular features that synapomorphies among superkingdoms are hard to identify. This is true of molecular sequences as well as features of cell shape and internal structure.
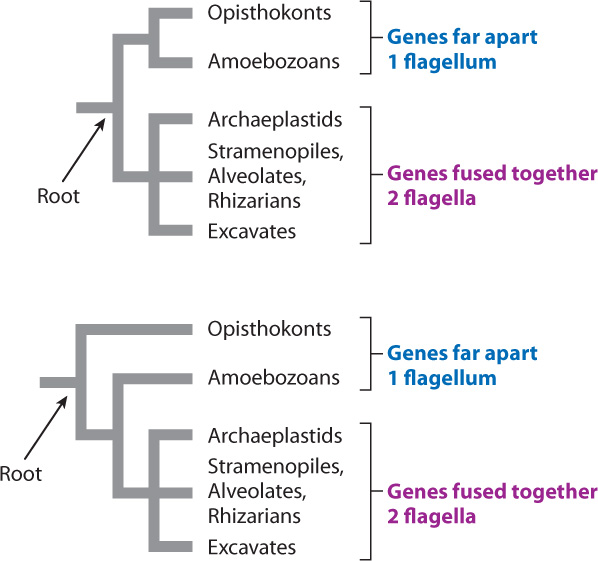
A leading hypothesis for sorting the superkingdoms reflects a seemingly minor feature of gene organization, but one that has so far been successful in dividing known eukaryotes into two groups. In opisthokonts and amoebozoans, genes that code for the enzymes dihydrofolate reductase and thymidylate synthase do not lie next to each other on a single chromosome. In all other eukaryotes, the two genes are fused together along one chromosome. As the two genes are also separate in bacteria, this observation suggests that the gene fusion observed in many eukaryotes is a synapomorphy that unites most eukaryotes and separates them from the branch we share with fungi and amoebozoans. Other lines of evidence support this interpretation, particularly the observation that opisthokont and amoebozoan cells have only one flagellum, but other eukaryotes (if they have them at all) usually have two or more.
This being the case, we can draw the tree in two different ways, as shown in Fig. 27.22. The hypothesis that opisthokonts and amoebozoans are sisters and that these two groups together are sister to all other eukaryotes (the top tree) is consistent with the data on gene location and flagella number. But so is an alternative hypothesis—that the opisthokonts are sister to all other eukaryotes, with gene fusion occurring near the base of the limb that contains archaeplastids, stramenopiles, alveolates, rhizarians, and excavates (the bottom tree). At present, we lack data to choose between these hypotheses, but the resolution of branching pattern in eukaryotic phylogeny will help us understand which features were present in the last common ancestor of living eukaryotes and how the immense diversity of eukaryotic organisms evolved from those humble beginnings.