28.3 HOW TO BUILD A MULTICELLULAR ORGANISM
We have emphasized three general requirements for complex multicellular life: Cells must stick together; they must communicate with one another; and they must participate in a network of genetic interactions that regulates cell division and differentiation. Once these are in place, the stage is set for the formation of specialized tissues and organs observed in plants, animals, and other complex multicellular organisms.
28.3.1 Complex multicellularity requires adhesion between cells.
If a fertilized egg is to develop into a complex multicellular organism, it must divide many times, and the cells produced from those divisions must stick together. Moreover, they must retain a specific relationship with one another in order for the developing organism to function. Chapter 10 introduced the molecular mechanisms that hold multicellular organisms together. We begin by reviewing those mechanisms briefly before placing them in evolutionary context.
In animals, proliferating cells commonly become organized into sheets of cells called epithelia that line the inside and cover the outside of the body. Within epithelia, adjacent cells adhere to one another by means of transmembrane proteins, especially cadherins, that form molecular bonds between cells. Epithelial cells also secrete an extracellular matrix made of proteins and glycoproteins and attach themselves to this matrix by means of other transmembrane proteins called integrins. Cadherins, integrins, and additional transmembrane proteins thus provide the molecular mechanisms for adhesion in animals (Chapter 10).
Cadherins and integrins do not occur in other complex multicellular organisms, but all such organisms require some means of keeping their cells stuck together. How do they do it? Plants also synthesize adhesive molecules that bind cells into tissues, but in this case the molecules are polysaccharides called pectins (pectins are what make fruit jelly jell). Molecules that control adhesion between adjacent cells are less well known in other complex multicellular organisms, but the general picture is clear. Without adhesion there can be no complex multicellularity, and different groups of eukaryotes have employed distinct types of molecular “glue” for this purpose.
28-7
28.3.2 How did animal cell adhesion develop?
The genome of the choanoflagellate Monosiga brevicollis provides fascinating insight into the evolution of adhesion molecules. Choanoflagellates, the closest protistan relatives of animals, are unicellular microorganisms. Therefore, it came as a surprise that the genes of M. brevicollis code for many of the same protein families that promote cell adhesion in animals. The choanoflagellate genome contains genes for both cadherin and integrin proteins. Clearly, these proteins are not supporting epithelia in M. brevicollis, so what are they doing?
One approach to an answer came from the observation that cells stick not only to one another but also to their substrates. Proteins that originally evolved to promote adhesion to sand grains or a rock surface may have been co-opted by evolution for cell–cell adhesion. It is also possible that adhesion molecules originally facilitated the capture of bacterial cells, improving predation by causing prey to adhere to the predator.
An intriguing clue to this problem comes from careful laboratory studies of choanoflagellates (Fig. 28.7). Although choanoflagellates are fundamentally unicellular, simple multicellular structures can be induced in a number of species. Multicellularity is induced by molecular signals, and, unexpectedly, the source of these signals is a bacterium, the preferred prey of the choanoflagellates. When they detect their food bacteria, the choanoflagellates form a novel multicellular structure. The function of adhesion molecules in these choanoflagellates has not yet been determined, but these observations support the hypothesis that they facilitate predation.
FIG. 28.7: How do bacteria influence the life cycles of choanoflagellates?
BACKGROUND Choanoflagellates are the closest protistan relatives of animals, so an understanding of cell adhesion, cell differentiation, and multicellularity in these organisms stands to illuminate early events in the evolution of animal multicellularity. Nicole King and her colleagues cultured the choanoflagellate species Salpingoeca rosetta in the laboratory. High-speed videos revealed that these choanoflagellates differentiate into several distinct cell types in the course of their life cycle. Actively swimming cells can be fast swimmers or slow swimmers that differ in shape and ornamentation (Fig. 28.7a). When swimming cells make contact with a solid surface, they can differentiate a stalk that tethers them to the substrate (Fig. 28.7b). Simple multicellular colonies in the shape of rosettes (Fig. 28.7c) were also seen when the choanoflagellates were cultured in the presence of many bacteria, including Algoriphagus machipongonesis.
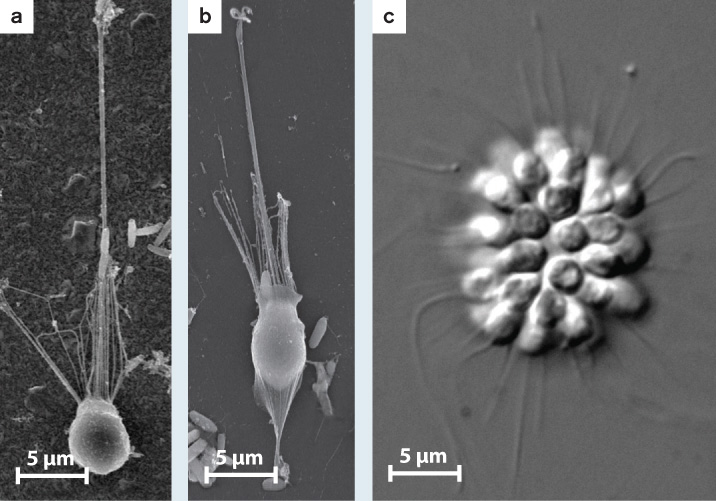
Salpingoeca rosetta choanoflagellates can exist as single cells that swim through their watery environment (a); they can be stalked cells that live attached to surfaces (b); or, in the presence of specific bacteria, they can form simple multicellular structures (c).
HYPOTHESIS The bacterium Algoriphagus machipongonesis induces the choanoflagellate to form rosette-like colonies.
EXPERIMENT The scientists grew the chonaoflagellates in a medium containing only A. machipongonesis.
RESULTS The multicellular state formed (Fig. 28.7c).
CONCLUSION Choanoflagellates differentiate several cell types upon cues from the environment. In particular, molecular signals from prey bacteria can induce a simple form of multicellularity. The direct ancestors of animals may have gained simple multicellularity by a similar route.
FOLLOW-UP WORK Research is continuing to investigate the molecular mechanisms of cell differentiation, cell adhesion, and signal reception in these distant relatives of ours.
SOURCE Dayel, M., R. A. Alegado, S. R. Fairclough, T. C. Levine, S. A. Nichols, K. McDonald, and N. King. 2011. “Cell Differentiation and Morphogenesis in the Colony-Forming Choanoflagellate Salpingoeca rosetta.” Developmental Biology 357:73–82.
To date, cadherins have been found only in choanoflagellates and animals, but proteins of the integrin complex extend even deeper into eukaryotic phylogeny, occurring in single-celled protists that branch near the base of the opisthokont superkingdom (Chapter 27). Such a phylogenetic distribution provides strong support for the general hypothesis that cell adhesion in animals resulted from the redeployment of protein families that evolved to perform other functions before animals diverged from their closest protistan relatives.
28.3.3 Complex multicellularity requires communication between cells.
In complex multicellular organisms, it is not sufficient for cells to stick together. They must also be able to communicate. As discussed in Chapter 9, cells communicate by molecular signals. A signaling molecule (generally a protein) synthesized by one cell binds with a receptor molecule (also a protein) on the surface of a second cell, essentially flipping a molecular switch that activates or represses gene expression in the receptor cell’s nucleus. Plants and animals both have signaling pathways based on receptor kinases, and, as in the case of adhesion molecules, these signaling pathways, or components of the pathways, have also been identified in their close protistan relatives.
28-8
Evidently, many of the signaling pathways used for communication between cells in complex multicellular organisms first evolved in single-celled eukaryotes. Again we can ask, what function did molecular signals and receptors have in the protistan ancestors of complex organisms?
All cells have transmembrane receptors that respond to signals from the environment. In some cases, the signal is supplied by food organisms (such as the bacteria that induce simple multicellularity in choanoflagellates), and in some cases the cells sense nutrients, temperature, or oxygen level. Single-celled eukaryotes also communicate with other cells within the same species, not least to ensure that two cells can find each other to fuse in sexual reproduction. Signaling between two cells within an animal’s body can be seen as a variation on this more general theme of cellular responses to other cells and the physical environment.
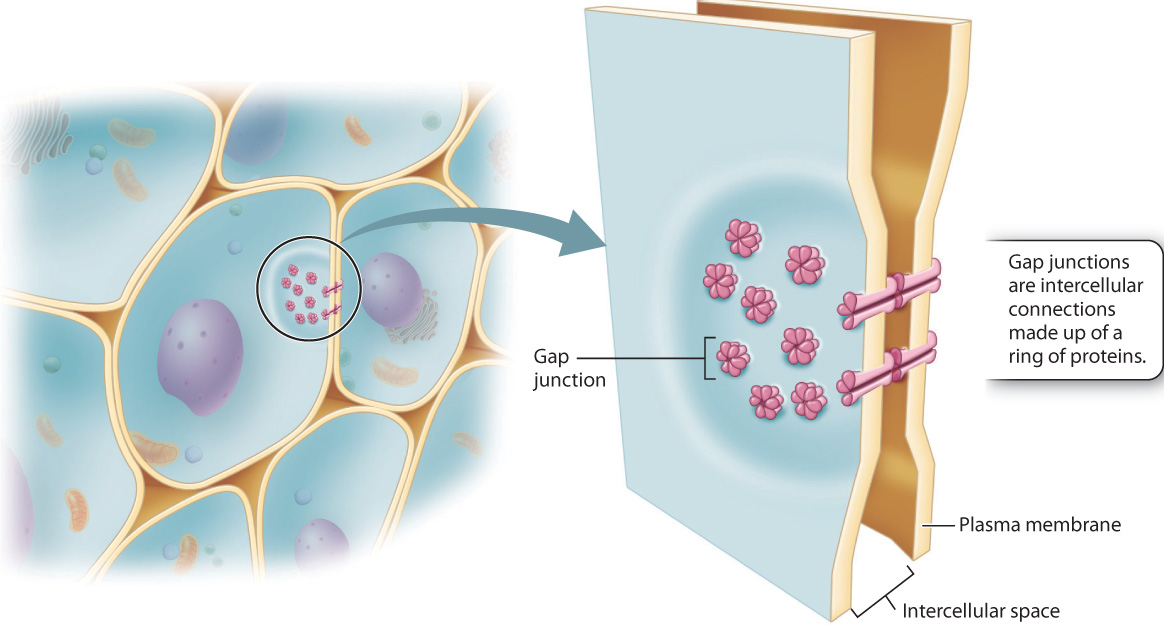
While all eukaryotic cells have molecular mechanisms for communication between cells, complex multicellular organisms have distinct cellular pathways for the movement of molecules from one cell to another. For example, animals more complex than sponges have gap junctions, protein channels that allow ions and signaling molecules to move from one cell into another (Fig. 28.8). Gap junctions not only help cells to communicate with their neighbors, they allow targeted communication between a cell and only one or a few of the cells that surround it (Chapter 10).
Complex photosynthetic organisms, in contrast, have an intrinsic barrier to intercellular communication: the cell wall. Careful observation of plant cell walls using the electron microscope reveals that these walls have tiny holes through which thin strands of cytoplasm extend from one cell to the next (Fig. 28.9). Called plasmodesmata, these intercellular strands are lined by extensions of the cell membrane and contain tubules connecting the endomembrane systems of the two cells. Like gap junctions, plasmodesmata permit signaling molecules to pass between cells in a targeted fashion.
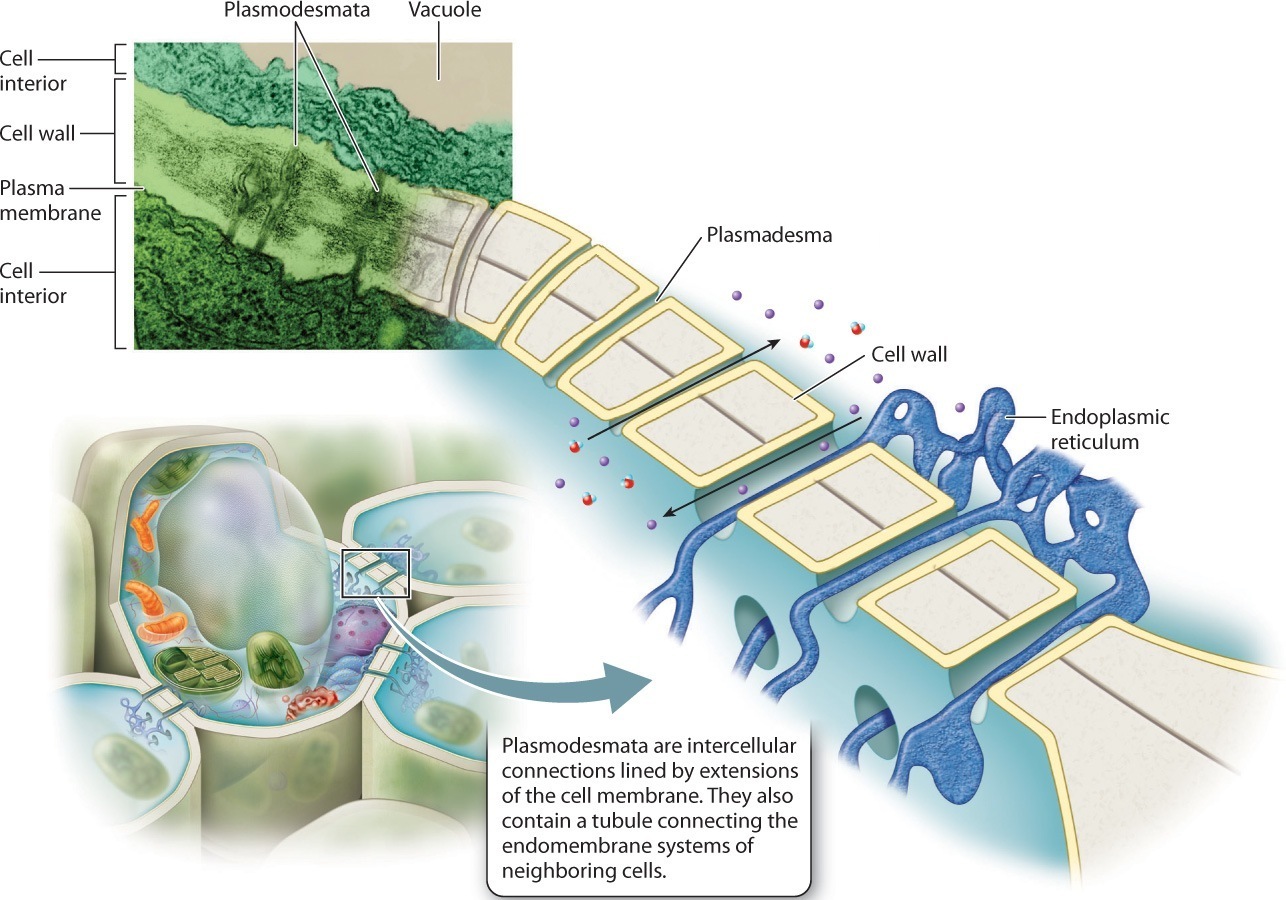
Other complex multicellular organisms also have channels that connect adjacent cells. As similar channels do not occur in most other eukaryotic organisms, they appear to represent an important step in the evolution of complex multicellularity.
28.3.4 Complex multicellularity requires a genetic program for coordinated growth and cell differentiation.
All of the cells in your body derive from a single fertilized egg, and most of those cells contain the same genes. Yet your body contains about 200 distinct cell types precisely arranged in a variety of tissues and organs. How can two cells with the same genes become different cell types? The answer lies in development, the system of gene regulation that guides growth from zygote to adult (Chapter 20).
Development is the product of molecular communication between cells. Cells have different fates depending on which genes are switched off or on by the molecular signals they receive. Different signals induce the reorganization of cytoskeletons and alter the production of proteins. As a result, a stem cell may become an epithelial cell, or a muscle cell, or a neuron. This observation leads to another question: What causes the same gene to be turned on in one cell and off in another? The ultimate answer is that two cells in the same developing organism can be exposed to very different environments.
28-9
When we think about development as a process of programmed cell division and differentiation, the link to unicellular ancestors becomes clearer. Many biological innovations accompanied the evolution of complex multicellularity, but, as noted in Chapter 27, the differentiation of distinct cell types is not one of them. Many unicellular organisms have life cycles in which different cell types alternate in time, depending on environmental conditions. For example, if we experimentally starve dinoflagellate cells, two cells will undergo sexual fusion to form morphologically and physiologically distinct resting cells protected by thick walls. When food becomes available again, the cells undergo meiotic cell division to form new feeding cells. Many other single-celled eukaryotes differentiate resting cells in response to environmental cues, especially deprivation of nutrients or oxygen.
The innovation of complex multicellularity was to differentiate cells in space instead of time. In a three-dimensional multicellular organism, only surface cells are in direct contact with the outside environment. Interior cells are exposed to a different physical and chemical environment because the availability of nutrients, oxygen, and light decreases with increasing depth within tissues. In effect, there is a gradient of environmental signals within multicellular organisms. It is, therefore, possible that in the earliest organisms with three-dimensional multicellularity, a nutrient or oxygen gradient triggered differentiation of interior cells, causing cell differentiation of oxygen-or nutrient-starved interior cells much as these conditions induce the expression and repression of specific genes in their single-celled relatives. With increasing genetic control of cellular responses to signaling gradients, the seeds of complex development were sown.
A fascinating example supporting this hypothesis has been reported from research on green algae. The simple multicellular organism Volvox (see Fig. 27.15) has two types of cells, vegetative cells that photosynthesize and control movement of the organism, and reproductive cells. Cell differentiation in Volvox is regulated by a gene also involved in cell remodeling during the life cycle of Volvox’s single-celled relative Chlamydomonas, supporting the hypothesis that the spatial differentiation of cells in multicellular organisms began with the redeployment of genes that regulate cell differentiation in single-celled ancestors.
28-10
Bulk transport, discussed earlier with regard to the transport of nutrients and water within complex multicellular organisms, also affects developmental signaling. It increases the distance over which signals can travel, circumventing the limitations of diffusion and making possible spatially specific patterns of differentiation along the path of signal transport. For example, in animals the endocrine system releases hormones directly into the bloodstream, enabling them to affect cells far from those within which they formed (Chapter 38). Thus, the sex hormones estrogen and testosterone are synthesized in reproductive organs, but regulate development throughout the body, contributing to the differences between males and females.
The genome of the choanoflagellate Monosiga brevicollis, discussed earlier, has been a treasure trove of information on the antiquity of signaling molecules deployed in animal development. In addition to proteins that govern cell adhesion and epithelial cohesion in animals, M. brevicollis expresses a number of proteins active in animal cell differentiation. For example, signaling based on receptor kinases (Chapter 9), long thought to be restricted to animals, occurs in M. brevicollis. In other cases, individual domains of signaling proteins occur in the M. brevicollis genome, but not the complex multidomain proteins formed by animals. This is true, for example, of several protein complexes that are important in development. Similarly, molecules that play an important role in plant development are also being identified in the genomes of morphologically simple green algae. The key point is that genome sequences interpreted in light of eukaryotic phylogeny are now enabling biologists to piece together the patterns of gene evolution that accompanied the evolution of morphological complexity and diversity in plants and animals.