29.2 THE LEAF: ACQUIRING CO₂ WHILE AVOIDING DESSICATION
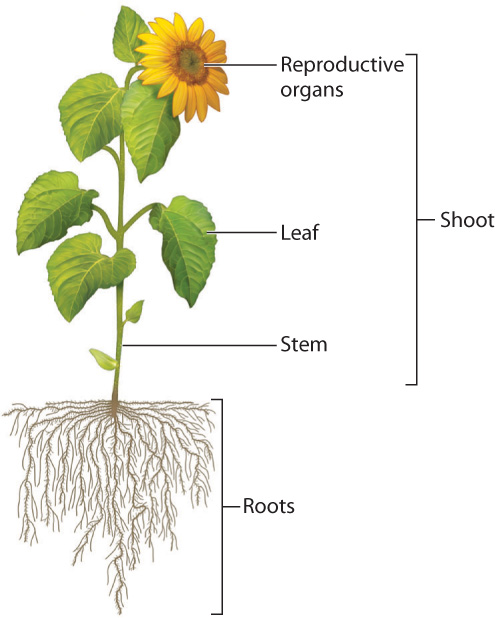
Fig. 29.3 shows the general features of a vascular plant. Aboveground, we see three major types of organ—leaves, stems, and reproductive organs—which collectively form the shoot. Roots, which are generally hidden from view in the ground, make up the fourth major organ system. We can answer the question of how vascular plants photosynthesize on land by examining how leaves, stems, and roots work together.
29-3
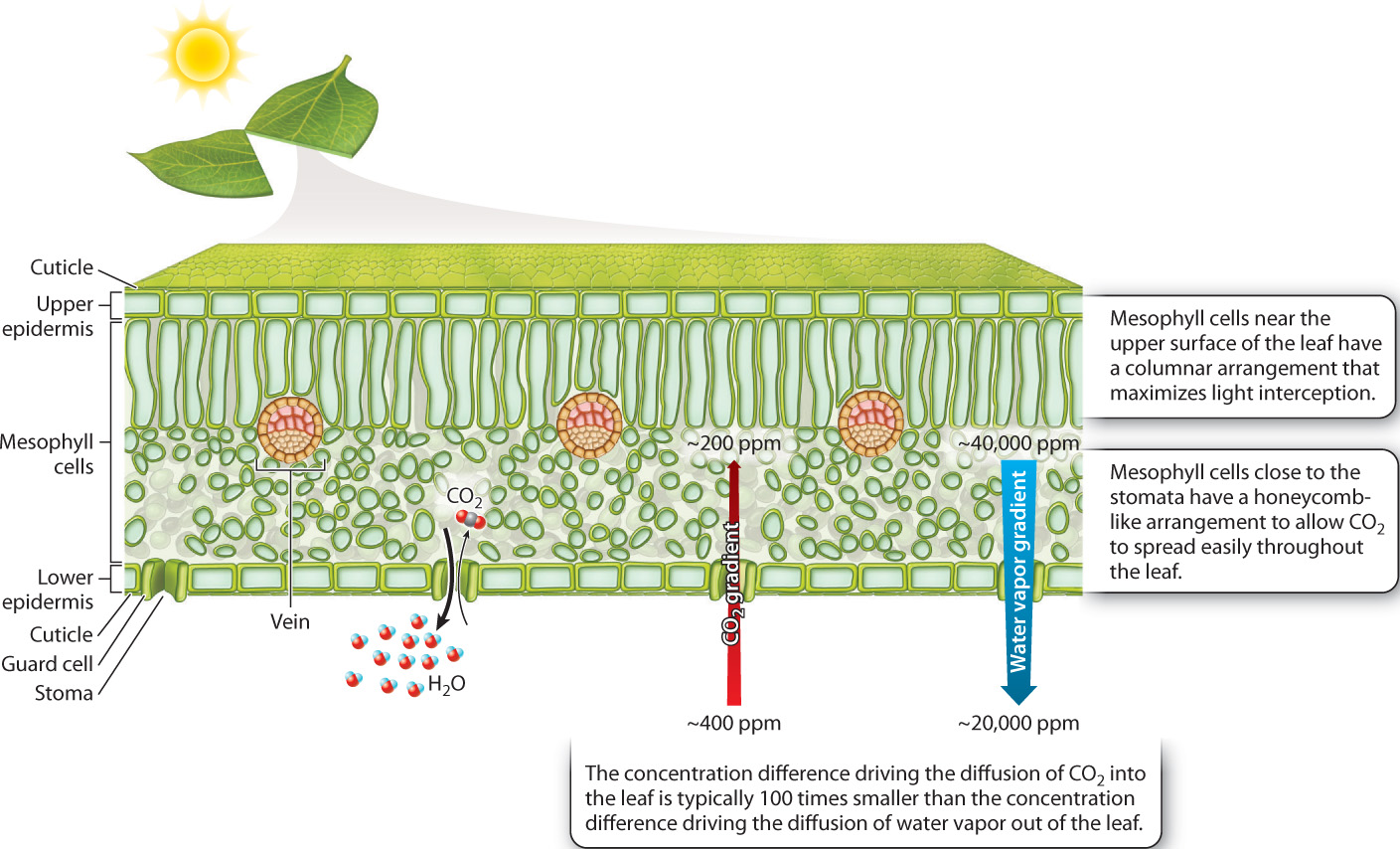
The leaf is the principal site of photosynthesis in vascular plants. A cross section shows the leaf’s three major tissues: sheets of cells called the epidermis that line the leaf’s upper and lower surfaces; loosely packed photosynthetic cells that make up the mesophyll (literally, “middle leaf”); and veins, the system of vascular conduits that connects the leaf to the rest of the plant (Fig. 29.4). We discuss how water and carbohydrates are transported through the vascular system in sections 29.3 and 29.4. In this section, we explore how the structure of leaves allows vascular plants to photosynthesize in air without drying out.
29.2.1 CO₂ uptake results in water loss.
Water is the resource that most often limits a plant’s ability to grow and function. Water availability is the single most important factor limiting agricultural yields, and rainfall patterns have a major impact on the structure and productivity of natural ecosystems (Chapter 47). Water has such a significant effect on the growth and functioning of plants because plants use large amounts of water. Why do plants require so much water?
The answer is not because water is consumed in supplying electrons for photosynthesis (Chapter 8). That process accounts for less than 1% of the water required by vascular plants. Instead, most of a plant’s need for water arises as a consequence of CO2 uptake in air.
As we saw in Chapter 25, CO2 is a minor constituent of air, about 400 parts per million at present. This low concentration limits the rate at which CO2 can diffuse into the leaf. Therefore, how fast a plant can take up CO2 depends in large part on the degree to which leaves can expose their photosynthetic cells to the surrounding air. If we look inside a leaf, we see that each mesophyll cell is largely surrounded by air. The mesophyll cells obtain the CO2 that they need for photosynthesis directly from these air spaces. If the air spaces within the leaf were completely sealed off, photosynthesis would quickly run out of CO2 and come to a halt. But they’re not sealed off—the leaf’s air spaces are connected to the air surrounding the leaf by pores in the epidermis. As photosynthesis draws down the concentration of CO2 molecules within the leaf’s air spaces relative to the concentration of CO2 in the outside air, the difference in concentration between the inside and the outside of the leaf causes CO2 to diffuse into the leaf, replenishing the supply of CO2 for photosynthesis.
29-4
The ability of leaves to draw in CO2 comes at a price, however: If CO2 can diffuse into the leaf, water vapor can diffuse out (Fig. 29.4). Furthermore, the rate at which water vapor diffuses out of a leaf is much greater than the rate at which CO2 diffuses inward. Molecules diffuse from regions of higher concentration to regions of lower concentration, and the rate of diffusion is proportional to the difference in concentration. On a sunny summer day, the difference in water vapor concentration between the air spaces within a leaf and the air outside can be more than 100 times larger than the difference in concentration of CO2. Add the fact that water is lighter than CO2, and so diffuses 1.6 times faster for the same concentration gradient, and it becomes clear why, on a sunny summer day, several hundred water molecules are lost for every molecule of CO2 acquired for photosynthesis.
Because of this difference, the water costs associated with photosynthesis in air are large. A sunflower leaf, for example, can lose an amount equal to its total water content in as little as 20 minutes. To put this rate of water loss in perspective, you would have to drink about 2 liters per minute to survive a similar rate of loss. Vascular plants can sustain such high rates of water loss because they can access the only consistently available source of water on land: the soil. The evaporative loss of water vapor from leaves, commonly called transpiration, is the end point of the movement of water from the soil, through the bodies of vascular plants, and then, as water vapor, into the atmosphere. Therefore, the challenge of keeping photosynthetic cells hydrated is met partly by the continual supply of water from the soil and partly by limiting the rate at which water already in leaf tissues is lost to the atmosphere.
Question Quick Check 1
6HOHpgyfelO7/73jOZiz0VPt9IQ1yYCc4BkIXg==29.2.2 The cuticle restricts water loss from leaves but inhibits the uptake of CO₂.
Epidermal cells secrete a waxy cuticle on their outer surface that limits water loss. Without a cuticle, the humidity within the internal air spaces would drop and the photosynthetic mesophyll cells would dry out. Interestingly, it is the evaporation of water from the exterior face of epidermal cells that brings waxes secreted by the cell’s plasma membrane up to the surface of the cell wall. There the wax molecules self-organize into a protective layer that builds up until water loss essentially ceases. This process of cuticle formation ensures the rapid repair of any damage to the cuticle that would allow water to evaporate. The mechanism is far from perfect, however, because the cuticle prevents CO2 from diffusing into the leaf even as it restricts water vapor from diffusing out of it.
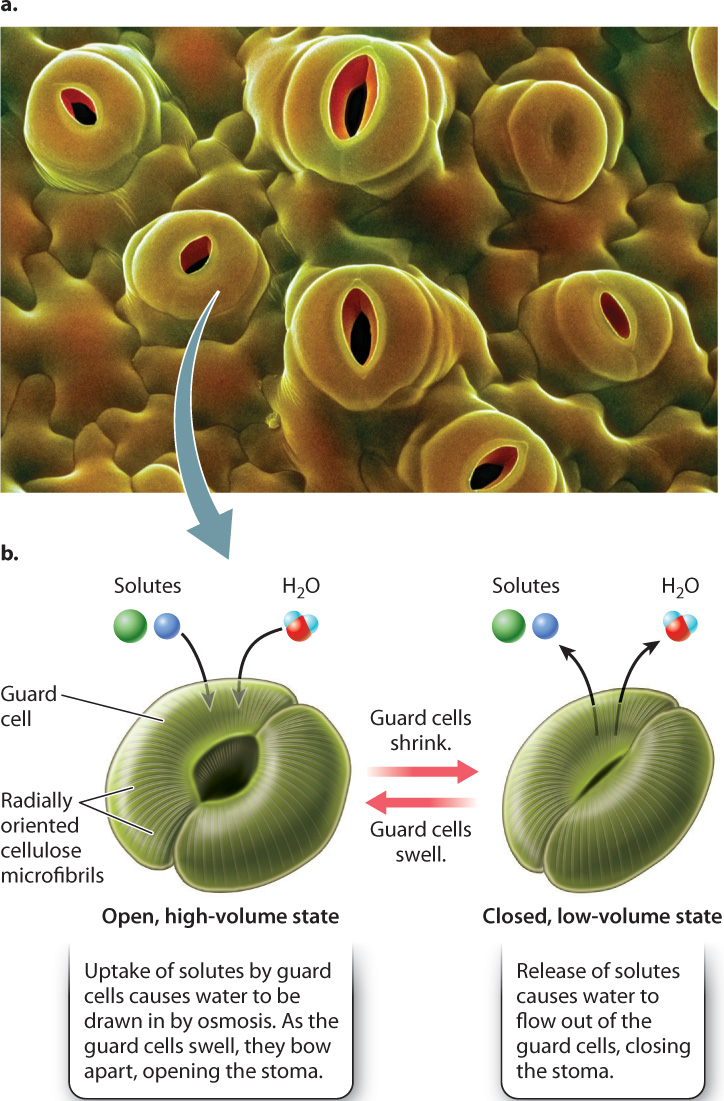
As noted above, small pores in the epidermis allow CO2 to diffuse into the leaf (Fig. 29.5a). These pores, called stomata (singular, stoma), regulate the diffusion of gases between the interior of the leaf and the atmosphere. Stomata can be numerous, up to 1000 per mm2. Yet because each one is small, less than 1% to 2% of the leaf surface is actually covered by these pores. Thus, even with stomata, the epidermis is a significant barrier to the diffusion of CO2 and water vapor.
The epidermis with its stomatal pores represents a compromise between the challenges of providing food (CO2 uptake) while preventing thirst (water loss). However, because both the dryness of the air and the wetness of the soil are variable, leaves must be able to alter the porosity of the leaf epidermis to maintain a balance between the rates of water loss to the atmosphere and water delivery from the soil.
29-5
29.2.3 Stomata allow leaves to regulate water loss and carbon gain.
Stomata are more than just holes in the leaf epidermis; they are hydromechanical valves that can open and close. Each stoma consists of two guard cells surrounding a central pore. The guard cells can shrink or swell, changing the size of the pore between the guard cells (Fig. 29.5b). How does this valve system work?
Cellulose microfibrils in the guard cell walls are oriented radially, that is, wrapped around the cell. The radially oriented microfibrils make it easier for swelling guard cells to expand in length rather than in girth. Because the guard cells are firmly connected at their ends, this increase in length causes them to bow apart, opening the stoma. Conversely, a decrease in cell volume causes the pore to close.
Guard cells control their volume by altering the concentration of solutes, for example the ions K+ and Cl–, in their cytoplasm. An increase in solute concentration causes water to flow into the cell by osmosis, whereas a decrease in solutes causes water to flow out of the cell. Recall from Chapter 5 that osmosis is the diffusion of water across a semipermeable membrane, such as the cell’s plasma membrane, from a region of higher water concentration to a region of lower water concentration. An increase in the concentration of solutes in guard cells is accompanied by a corresponding decrease in the concentration of water molecules. Thus, as guard cells add solutes, water diffuses into the cells, causing them to swell.
Stomata close by the same process, only in reverse. A decrease in solute concentration results in a higher concentration of water molecules inside a guard cell relative to the solution in the surrounding cell wall space, and water diffuses out of the cell, causing it to shrink.
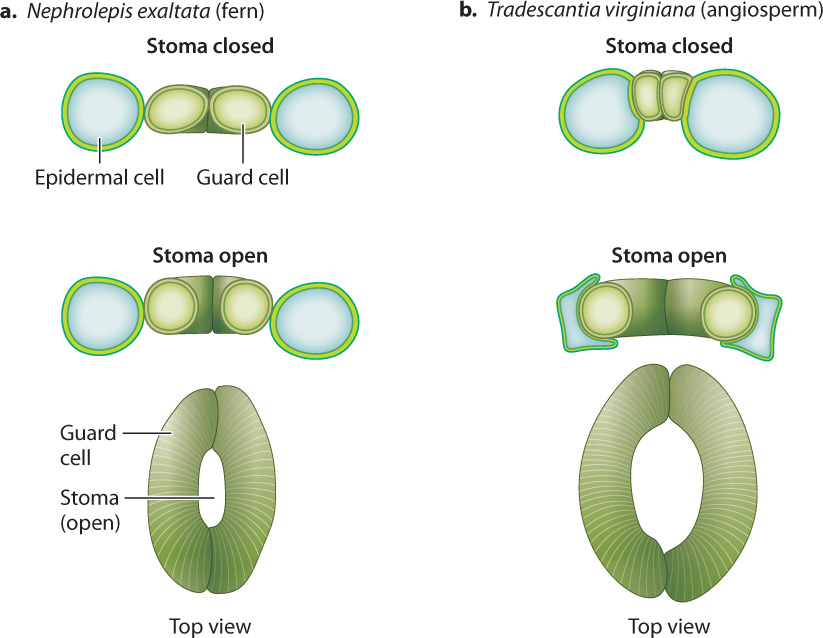
The accumulation of solutes inside guard cells requires an expenditure of energy in the form of ATP, which drives the uptake of solutes across the plasma membrane (Chapter 5). Closing, in contrast, does not require direct energy expenditure. Thus, the default or resting state of guard cells is “closed”; this is the state that conserves the hydration of the leaf. In angiosperms (the flowering plants), K+ and Cl– ions are transferred between guard cells and an adjacent epidermal cell, so an increase in the volume of one cell type is accommodated by a decrease in the other. This reciprocal change in cell size allows the stomatal pore to open much wider than it could if the guard cells operated on their own (Fig. 29.6). It takes approximately 10 minutes for angiosperm stomata to go from fully closed to fully open. During this time, the volume of each guard cell can increase as much as threefold.
To function effectively, stomata must open and close in response to both CO2 demand and water loss. Stomata are thus key sites for the processing of physiological information. Light stimulates stomatal opening, while high levels of CO2 inside the leaf (a signal that CO2 is being supplied faster than photosynthesis can take it up) cause stomata to close. When the soil can’t supply enough water to keep up with evaporation from the leaf, guard cells become dehydrated and shrink in volume, closing the stomata. Guard cell volume also changes in response to signaling molecules. For example, abscisic acid, a hormone produced during drought, causes stomata to close. This signal allows leaves to close their stomata early enough to prevent dehydration, rather than responding only when water has already been lost. Clearly, then, stomata are a key innovation in the evolution of land plants. Not surprisingly, fossils indicate that stomata evolved early in the colonization of land.
Before leaving the topic of how stomata affect rates of water loss from leaves, we pause to consider the following question: Is there any evidence that plants control the temperature of their leaves by regulating transpiration? The answer, perhaps surprisingly, is no. Although our own experience leads us to think of evaporation (for example, sweating) as a means of preventing overheating, plants transpire when their leaves are too hot or too cold. When leaves are above their optimal temperature for photosynthesis, cooling through transpiration is beneficial. But when temperatures are below the optimum, transpiration results in leaf temperatures that are even less favorable. In addition, the hot environments in which evaporative cooling would be beneficial often coincide with low availability of water in the soil. For this reason, plants in arid regions tend to rely on mechanisms other than evaporative cooling, such as reflective hairs and waxes and small leaf size, to prevent their leaves from overheating.
29-6
29.2.4 CAM plants use nocturnal CO₂ storage to avoid water loss during the day.
In most plants, water loss and photosynthesis both peak at the same time, when the sun is high overhead. What if a plant could open its stomata to capture CO2 at night, when cool air limits rates of evaporation?
In fact, a number of plants have evolved precisely this mechanism, called crassulacean acid metabolism, or CAM, to help balance CO2 gain and water loss (Fig. 29.7). CAM (named after the Crassulaceae family of plants, which uses this pathway), provides a system for overnight storage, converting CO2 into a form that will not diffuse away. The storage form of CO2 is produced by the activity of the enzyme PEP carboxylase, which combines a dissolved form of CO2 (bicarbonate ion, HCO3–) with a 3-carbon compound called phosphoenol pyruvate (PEP). The resulting product is a 4-carbon organic acid that is stored in the cell’s vacuole.
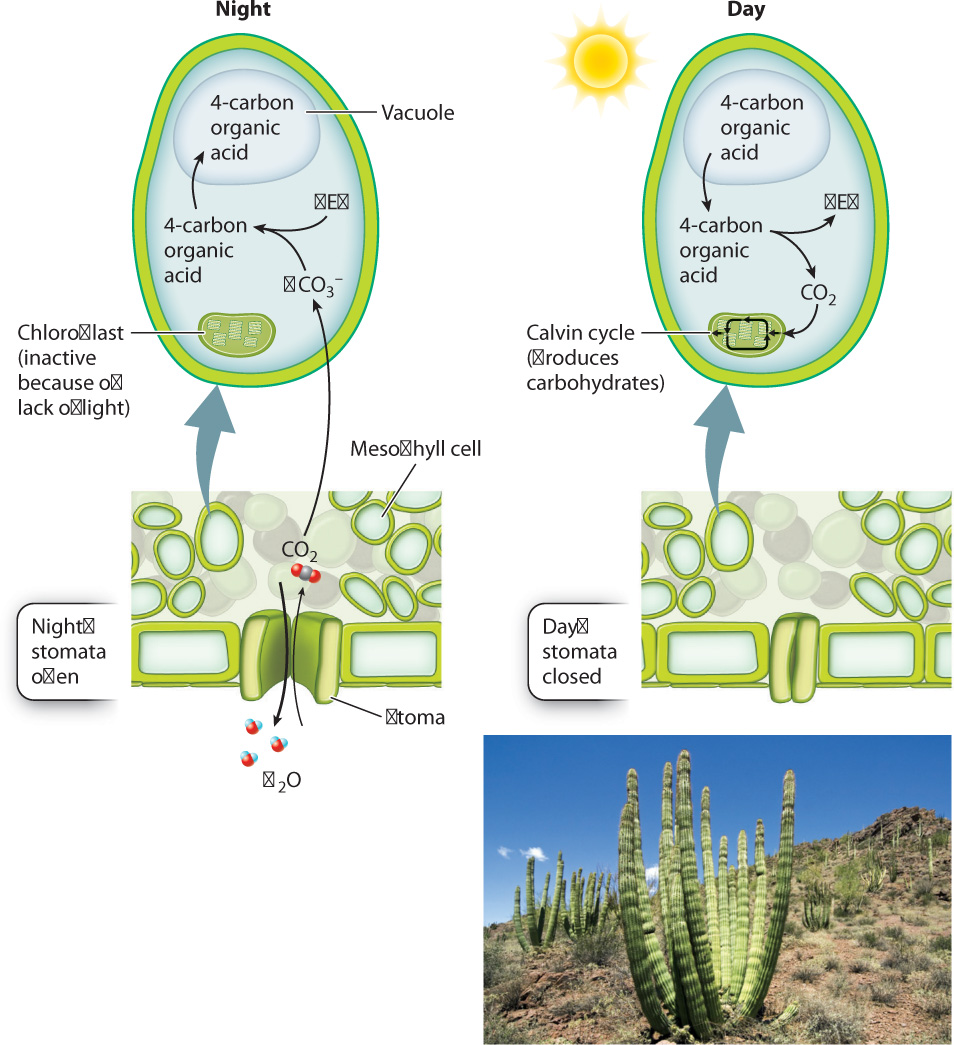
When the sun comes up the next morning, the stomata close, conserving water. At the same time, the 4-carbon organic acids are retrieved from the vacuole and decarboxylated—that is, their CO2 is released into the cytoplasm. Because the stomata are now closed, this newly released CO2 does not escape from the leaf. Instead, it diffuses into the chloroplast, where it can become incorporated into carbohydrates by the Calvin cycle. As we saw in Chapter 8, the Calvin cycle requires a continual supply of energy in the form of ATP and NADPH and so can operate only in the light.
The buildup of organic acid during the night results in a marked decrease in the pH of vacuoles. In 1815, the British naturalist Benjamin Heyne wrote that some of the plants in his garden in India tasted “as acid as sorrel” early in the morning, but by afternoon the acid taste had disappeared.
The nocturnal opening of stomata exhibited by CAM plants greatly increases the amount of CO2 gain per unit of water loss. Indeed, the CO2 : H2O exchange ratio for CAM plants is in the range of 1 : 50, nearly 10 times higher than that of plants that open their stomata during the day. However, CAM has a drawback. The rates of photosynthetic carbohydrate production by CAM plants tend to be low because ATP is needed to drive the uptake of organic acids into the vacuole and because only so much organic acid can accumulate in the vacuole. CAM is therefore most common in habitats such as deserts, where water conservation is crucial, and among epiphytes, plants that grow high in the canopy of other plants, without contact with the soil.
Because CAM uses enzymes (for example, PEP carboxylase) and compartments (for example, vacuoles) that exist in all plant cells, it is not surprising that it has evolved multiple times. CAM occurs in all four groups of vascular plants shown in Fig. 29.1. It is most widespread in the angiosperms, occurring in 5% to 10% of species. Vanilla orchids and Spanish moss, two well-known epiphytes, as well as many cacti, exhibit CAM.
29-7
29.2.5 C₄ plants suppress photorespiration.
Photorespiration adds another wrinkle to the challenge of acquiring CO2 while minimizing water loss. Recall from Chapter 8 that either CO2 or O2 can be a substrate for rubisco, the key enzyme in the Calvin cycle. CO2 as a substrate leads to the production of carbohydrates through photosynthesis; O2 as a substrate results in a net loss of energy and the release of CO2, a process called photorespiration. (Photorespiration is similar to aerobic respiration only in the sense that it uses O2 and releases CO2. However, plants do not gain energy; they lose it.)
Photorespiration presents a significant challenge for land plants because air contains approximately 21% O2 but less than 0.04% CO2. Although rubisco reacts more readily with CO2 than with O2, the sheer abundance of O2 means that O2 is a substrate for rubisco some of the time. At moderate leaf temperatures, O2 is the substrate instead of CO2 as often as 1 time out of 4. At higher temperatures, O2 is even more likely to be the substrate for rubisco, and the rates of photorespiration are yet higher. The reason is that higher temperatures decrease both rubisco’s affinity for CO2 relative to O2 and the solubility of CO2 relative to O2 in water.
Some plants have evolved a way to reduce the energy and carbon losses associated with photorespiration. These are the C4 plants, which suppress photorespiration by increasing the concentration of CO2 in the immediate vicinity of rubisco. C4 plants take their name from the fact that they, like CAM plants, use PEP carboxylase to produce 4-carbon organic acids that subsequently supply the Calvin cycle with CO2. The Calvin cycle produces 3-carbon compounds (Chapter 8), and plants that do not use 4-carbon organic acids to supply the Calvin cycle with CO2 are thus referred to as C3 plants.
Both CAM and C4 plants produce 4-carbon organic acids as the entry point for photosynthesis. However, in CAM plants, CO2 capture and the Calvin cycle take place at different times; in C4 plants, they take place in different cells. It is the spatial separation between CO2 capture and the Calvin cycle that allows C4 plants to suppress photorespiration.
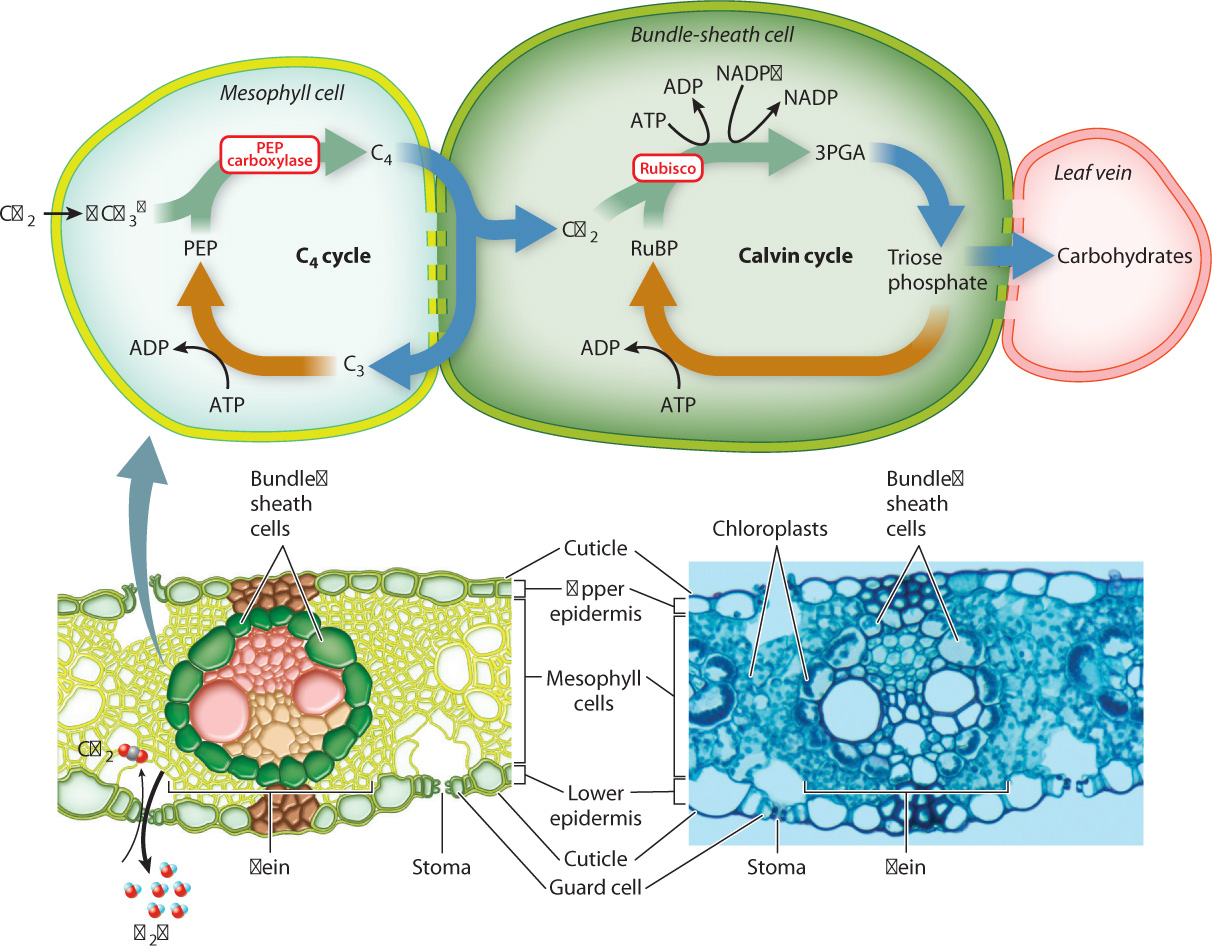
C4 plants intitially capture CO2 in mesophyll cells by means of PEP carboxylase, which combines a dissolved form of CO2 (bicarbonate ion, HCO3–) with the 3-carbon compound PEP. This produces 4-carbon organic acids that diffuse through plasmodesmata into the bundle sheath (Fig. 29.8), a cylinder of cells that surrounds each vein. Once inside bundle-sheath cells, the 4-carbon compounds are decarboxylated, releasing CO2 that is then incorporated into carbohydrates through the Calvin cycle (Chapter 8). The C4 cycle is completed as the 3-carbon molecules generated during decarboxylation diffuse back to the mesophyll, where ATP is used to re-form PEP. Because the C4 cycle operates much faster than the Calvin cycle, the concentration of CO2 within bundle-sheath cells builds up, reaching levels as much as five times higher than in the air surrounding the leaf. The increase in the concentration of CO2 in bundle-sheath cells makes it much less likely that rubisco will use O2 as a substrate.
29-8
C4 plants have high rates of photosynthesis because they do not suffer the losses in energy and reduced carbon associated with photorespiration (Fig. 29.9). In addition, C4 plants often have a more favorable CO2: H2O exchange ratio than plants that lack the ability to concentrate CO2. However, C4 photosynthesis has a greater energy requirement than conventional (C3) photosynthesis, as ATP must be used to regenerate PEP in the C4 cycle. Thus, C4 photosynthesis confers an advantage in hot, sunny environments where rates of photorespiration would otherwise be high. C4 photosynthesis has evolved as many as 20 times, but is most common among tropical grasses and plants of open habitats with warm temperatures. C4 plants include a number of important crops, especially maize (corn), sugarcane, and sorghum.
FIG. 29.9: How do we know that C₄ photosynthesis suppresses photorespiration?
BACKGROUND Studies using radioactively labeled CO2 showed that some species initially incorporate CO2 into 4-carbon compounds instead of the 3-carbon compounds that are the first products in the Calvin cycle. These C4 plants also have high rates of photosynthesis. Is this a new, more efficient photosynthetic pathway? Or do C4 plants have high rates of photosynthesis because they are able to avoid the carbon and energy losses associated with photorespiration?
HYPOTHESIS C4 plants do not exhibit photorespiration.
METHOD “Air tests,” as these experiments were first called, compared rates of photosynthesis in normal air (21% O2) and in an experimental gas mixture in which the concentration of O2 is only 1%. When the concentration of O2 is low, rubisco has a low probability of using O2 (instead of CO2) as a substrate, and thus photorespiration does not occur.
RESULTS
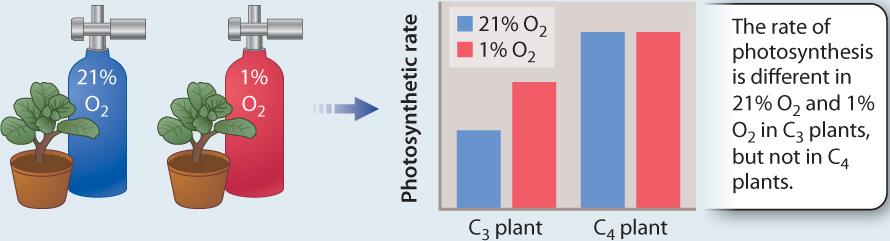
CONCLUSION Photosynthesis in C4 plants is not affected by differences in O2 concentration, indicating that significant photorespiration is not occurring in these plants. In contrast, the photosynthetic rate of the C3 plants increased in the low O2 environment, indicating that photorespiration depresses rates of photosynthesis in 21% O2.
FOLLOW-UP WORK The higher photosynthetic efficiency of C4 photosynthesis has prompted efforts, so far unsuccessful, to incorporate this pathway into C3 crops such as rice.
SOURCE Bjorkman, O., and J. Berry. 1973. “High-Efficiency Photosynthesis.” Scientific American 229:80–93.