31.2 PLANT HORMONES
A fundamental question in the development of multicellular organisms is what controls the differentiation of individual cells. In considering the formation of plant stems, we can ask: (1) What controls the arrangement of leaf primordia and the elongation of internodes? (2) What ensures that the differentiation of xylem and phloem cells produces a continuous vascular system extending from the roots to the leaves? (3) What determines whether an axillary bud remains dormant or develops into a new stem?
The answer, in each case, is that chemical signals guide the growth and differentiation of plant cells. Earlier, we discussed basic principles of cell signaling and communication (Chapter 9). Chemical signals play a central role in plant development because cell fate is determined by position. Some of the chemical signals important in plant development have highly localized effects. For example, signals that cause cells to express meristem identity genes are restricted to the region close to the shoot tip. Other signaling molecules, or their chemical precursors, enter the vascular system and are transported across the entire length of the plant. Because plants grow and develop in many places at once, signaling molecules that can move from one part of the plant to another play an important role in coordinating growth and development at the whole plant level.
31-7
31.2.1 Hormones affect the growth and differentiation of plant cells.
Hormones are chemical signals that influence both physiology and development. They play a central role in establishing and maintaining basic patterns of plant development, as well as coordinating growth in different parts of the plant in response to both internal and external environmental factors. A key feature of hormones is that they are effective in extremely low concentrations. In plants, hormones can be synthesized throughout the plant body, although they are most often produced in meristems. In addition, plant hormones can affect both the cells in which they are produced as well as cells located in distant regions of the plant.
Plant hormones influence cell growth and differentiation by altering patterns of gene expression as well as rates of cell division and cell expansion. How a cell responds to a particular hormone depends both upon its developmental stage and its position within the plant. In addition, the effect of any one hormone is often affected by the presence of one or more other hormones. This observation explains how a small number of hormones is able to shape the ongoing development and growth of plants.
31-8
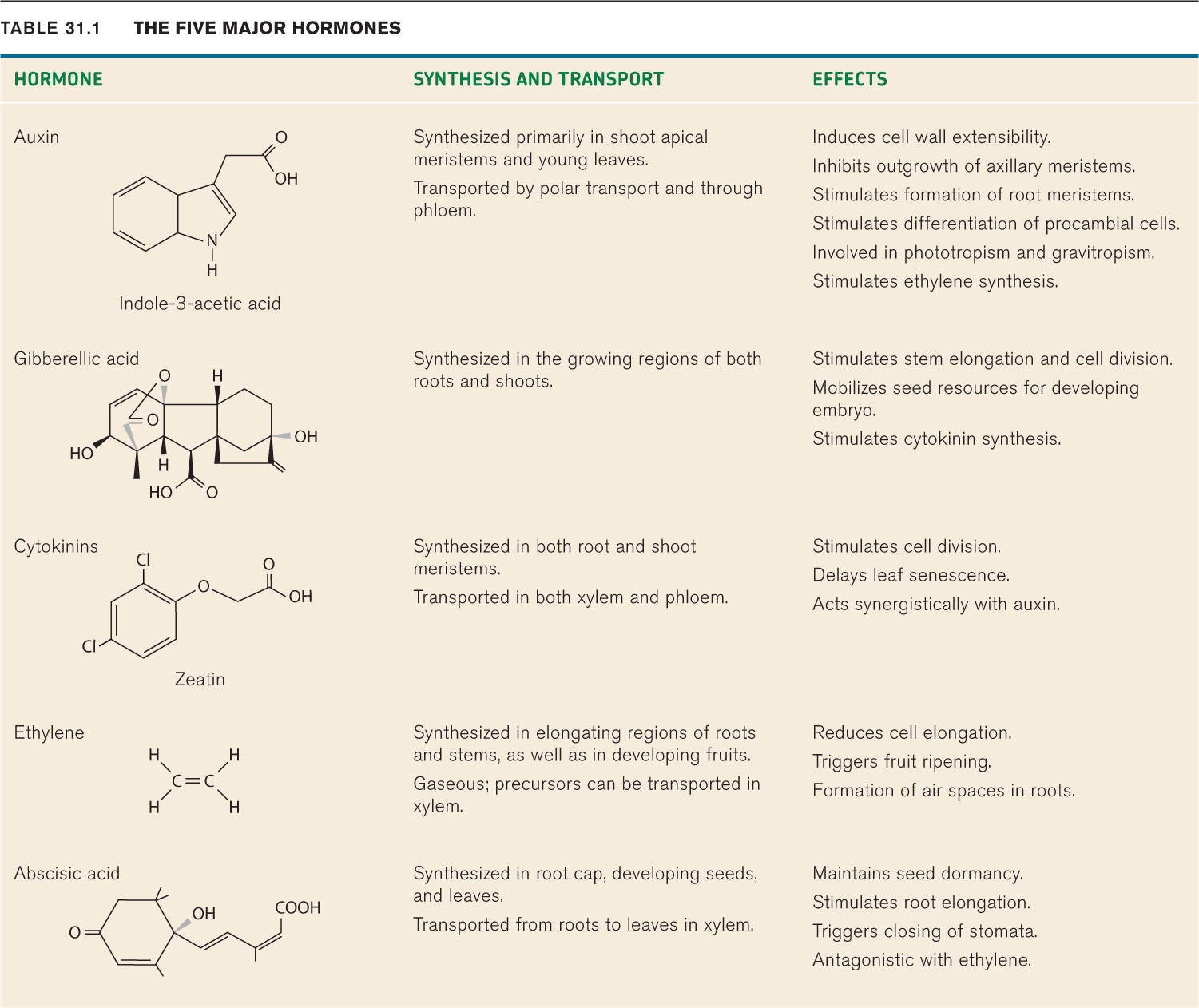
Traditionally, five major plant hormones have been recognized: auxin, gibberellic acid, cytokinins, ethylene, and abscisic acid (Table 31.1). One characteristic of these hormones is that each triggers a wide range of developmental effects. For example, ethylene can lead to fruit ripening (Chapter 30) and also slows the elongation of roots and stems. Several new chemicals with hormone-like properties have been recently discovered. Some of these have broad effects on plant development (e.g., strigolactone), whereas others appear to be more specific (e.g., florigen).
To illustrate how hormones influence plant growth and development, we consider three aspects of shoot development: the differentiation of vascular tissues, the elongation of internodes, and the formation of lateral branches.
31.2.2 Auxin transport guides the development of vascular connections between leaves and stems.
During the initial growth of leaf primordia, the resources needed can be met by diffusion. As the young leaves increase in size, their demand for water and carbohydrates quickly exceeds what can be supplied by diffusion. If they are to grow to even a fraction of their full size, leaves must establish vascular connections with the xylem and phloem in the stem.
The plant hormone auxin was first identified by its ability to cause shoots to elongate. However, auxin has an even more remarkable property: It is transported preferentially from young leaves to the differentiated vascular tissues in the stem. In this way, the movement of auxin creates a template that guides vascular differentiation.
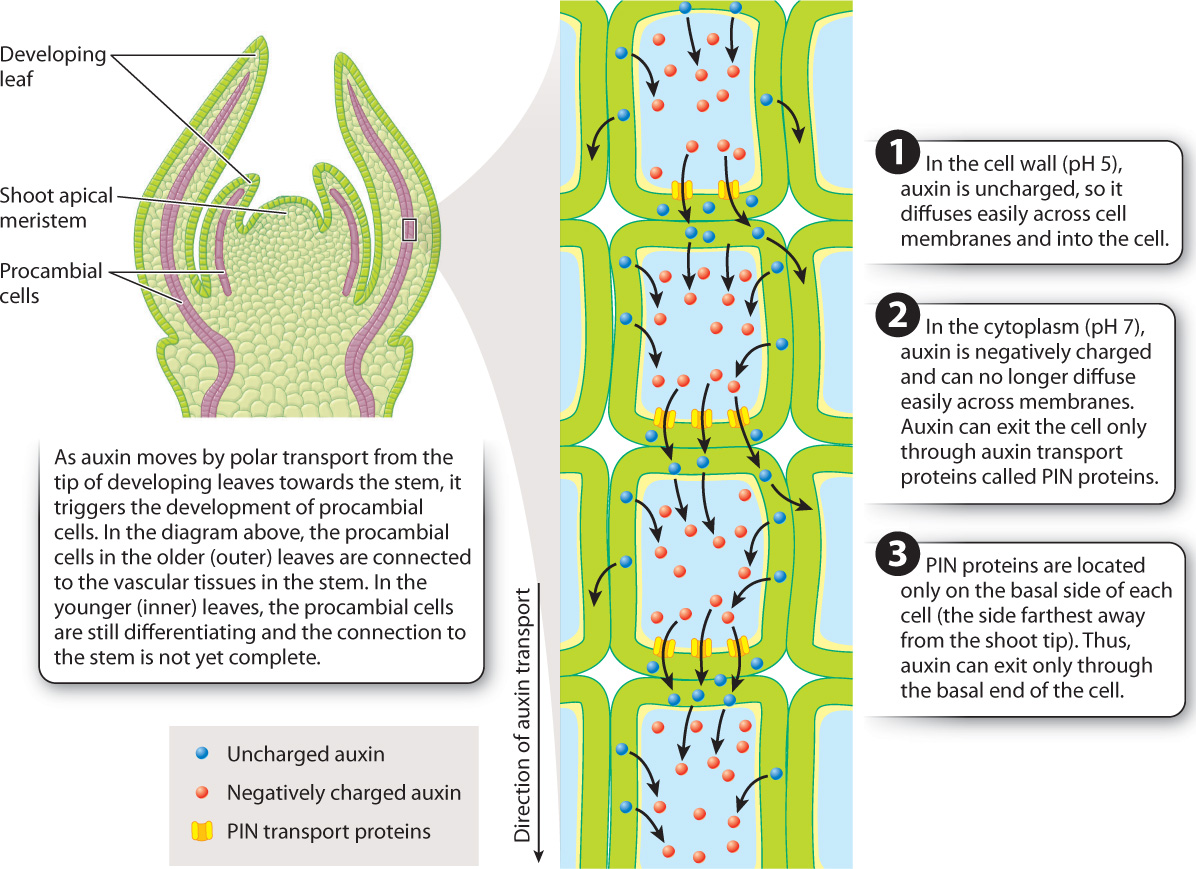
Auxin is synthesized and secreted by cells in young, expanding leaves. In the cell wall, auxin carries no net charge and thus can enter surrounding cells by diffusing across the plasma membrane. However, because the pH of the cytoplasm is higher than the pH of the cell wall, auxin loses a proton. As a result, auxin has a net negative charge when inside the cell, preventing it from moving easily across the plasma membrane. For auxin to leave the cell, a specific plasma membrane transport protein known as PIN is required. By restricting the placement of PIN proteins to only one face of the cell, plants can control the direction of auxin movement. Each cell in a plant’s body has an apical end and a basal end that are maintained independent of gravity or orientation. PIN proteins typically are located only on the basal side of cells, the side farthest away from the shoot tip. As a result, the prevailing direction of auxin movement is in the apical-to-basal (top-to-bottom) direction. The coordinated movement of auxin across many cells is referred to as polar transport (Fig. 31.9).
31-9
Auxin affects cells both by stimulating their elongation and increasing their production of PIN proteins. Thus, auxin transport results in linear rows of elongated cells as outflow from the basal side of one cell leads to enhanced auxin concentration in the cell immediately below. The elongated cells that carry auxin from the developing leaves to the stem differentiate to become procambial cells. Procambial cells retain the capacity for cell division and ultimately give rise to both xylem and phloem. The common origin of xylem and phloem from procambial cells explains why these two tissues are always found in close association throughout the length of the plant.
As auxin moves by polar transport out of developing leaves it is drawn towards the vascular tissues in the stem. The reason this happens is that auxin moves between cells by diffusion. Recall from Chapter 29 that molecules diffuse from regions of higher concentration to regions of lower concentration. The vascular tissues within the stem create a region of low auxin concentration because they transport auxin towards the roots faster than it can move through undifferentiated cells. In this way, the polar transport of auxin that originates in developing leaf primordia is responsible for establishing the vascular connections with the stem that allow leaves to grow and function.
The vascular system of an elongating stem is built from the successive addition of procambial strands that initiate in the developing leaves. The simplest arrangement, which is characteristic of the lycophytes, is a single cylinder of vascular tissues, with xylem at the center and phloem on the outside. The procambial strands that connect each leaf to the stem merge with this central cylinder.
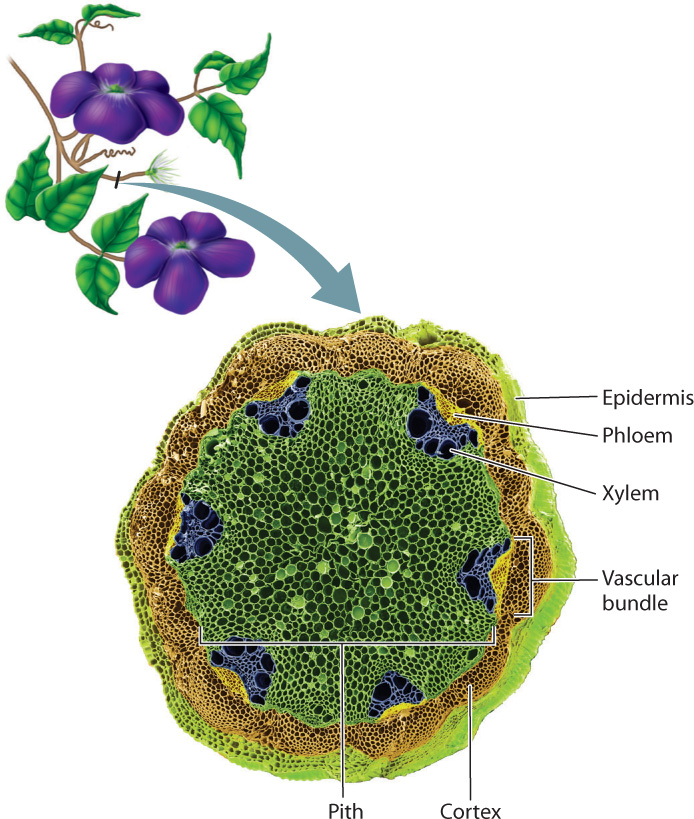
In seed plants, the most common configuration is a ring of vascular bundles near the outside of the stem. The bundles have xylem conduits toward the center of the stem and phloem nearer the outside of the stem (Fig. 31.10). Typically, each leaf develops vascular connections with several of the stem’s vascular bundles. The region between the epidermis and the vascular bundles is the cortex, and the region inside the ring of vascular bundles is the pith. The placement of the vascular bundles, which contain the stiff xylem conduits, near the outside of the stem increases the stem’s ability to support its leaves without bending over.
31.2.3 What is the developmental basis for the shorter stems of high-yielding rice and wheat?
Case 6 Agriculture: Feeding a Growing Population
In natural ecosystems, competition for sunlight creates a strong selective advantage for growing tall. Compare the slender vine in Fig. 31.1, which grows in a dense forest, with the compact barrel cactus that grows where there is little danger of being shaded by a neighboring plant. Whereas the ancestors of today’s crops competed for sunlight by growing taller, a key element of the high-yielding crop varieties developed during the Green Revolution is their shorter stems. These shorter-stemmed plants can support the much-larger seed mass induced by high rates of fertilizer application (Chapter 29). Moreover, by investing less in stems, these plants can allocate a greater percentage of their resources to reproduction.
At the time that the semidwarf varieties were developed, the underlying basis for their reduced stem growth was not known. What happens inside plants of these high-yielding crop varieties to make the stems shorter? Understanding how plants control intermodal elongation provides some insight.
The extent to which stems elongate can vary even within a single plant. For example, a tree in the temperate zone produces widely spaced green leaves during spring, but as autumn approaches the internodes cease elongating. As a result, bud scales become tightly packed together. This dramatic change in internode elongation is controlled by hormones.
Gibberellic acid is a plant hormone that stimulates the elongation of stems. It was first identified as a chemical produced by fungal pathogens that caused the stems of young rice plants to grow to many times their normal length. Giberellic acid increases internode elongation by reducing the force needed to cause cell walls to expand.
The genes responsible for reduced stem growth have now been identified in both wheat and rice. The semidwarf wheats have reduced sensitivity to gibberellic acid, and the shorter rice varieties are deficient in the biosynthetic enzymes needed to produce this hormone. In both cases, the result is shorter but sturdier plants.
31-10
31.2.4 Branching is affected by multiple hormones.
Branching allows plants to support more leaves, but producing branches too close together results in leaves that overlap and shade one another. What causes axillary buds to break dormancy and begin to grow? The answer to this question demonstrates how the interplay of different hormones can affect growth. In this case, the interplay of hormones produced in distinct parts of the plant communicates to axillary buds when conditions are right for the growth of new branches.
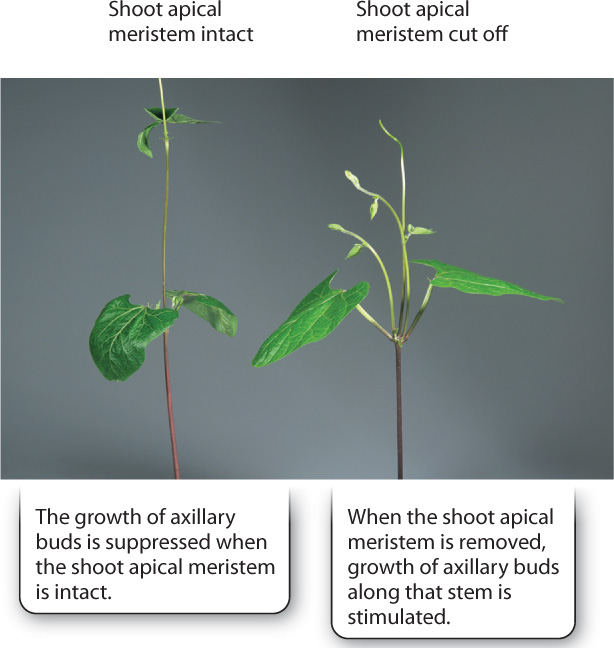
Removal of the shoot tip stimulates the outgrowth of axillary buds along that branch or stem (Fig. 31.11), suggesting that the shoot apical meristem somehow suppresses the growth of axillary buds. This suppression is referred to as apical dominance. If the shoot tip is cut off and a paste containing auxin is applied to the cut end of the stem, the axillary buds remain dormant. If instead the shoot tip is treated with a substance that inhibits auxin transport, branching is induced. Thus, branching is suppressed by the movement of auxin from the shoot tip toward the root. Apical dominance prevents branches from being formed too close to the shoot tip and also allows new branches to form should the shoot meristem be damaged.
Auxin suppresses the growth of axillary buds by inhibiting the synthesis of cytokinins, hormones that stimulate the outgrowth of axillary buds by increasing the rate of cell division (Table 31.1). When the apical meristem is removed, cytokinin synthesis increases in the stem nodes, triggering the growth of axillary buds. Recent studies indicate that this top-down control is complemented by the action of another hormone, strigolactone, which is made in roots and transported upward in the xylem. Plants that are unable to synthesize strigolactone produce many more branches than do wild-type plants. This indicates that strigolactone inhibits the outgrowth of axillary buds.
Clearly, the development of axillary buds is affected by multiple hormones, although the details of how they interact and under what conditions they are synthesized in greatest amounts are not yet known. One hypothesis is that strigalactones are transported to the shoot when the root system is unable to supply the water and nutrients required by new branches.