31.5 THE ENVIRONMENTAL CONTEXT OF GROWTH AND DEVELOPMENT
We take it for granted that shoots grow up and roots grow down. But if you lay a plant on its side, its stems will turn upward and its roots will bend downward. Similarly, if you place a houseplant near a window, the plant will bend over and grow toward the light. Plants are able to modify their growth in response to both gravity and light. They can sense and respond to the world around them in other ways as well. For example, trees planted close together produce stems that are taller and thinner than those of trees planted far apart. Is this simply what happens when crowded plants compete for the resources that they need to grow? No. Experiments have shown that the taller, thinner stems result from individual plants’ sensing the presence of their neighbors.
Plants gain information about the world around them in three principal ways. Photoreceptors, discussed in Chapter 30, provide information about the availability of light needed to drive photosynthesis. Receptors triggered by mechanical forces provide plants with information about physical influences such as gravity and wind. Chemical receptors allow plants to detect and respond to the presence of specific chemicals, as well as chemical gradients. When one of these sensory receptors is triggered, it sets in motion a chain of events that leads to a change in the way the plant develops and grows. Hormones play a key role in translating information gained by the plant’s sensory receptors into an appropriate developmental response.
31-17
31.5.1 Plants orient the growth of their stems and roots by light and gravity.
How do stems and roots control their orientation so that they grow in the right direction? In most cases, light provides the most useful signal to guide the growth of stems. Roots and seeds that germinate underground use several cues, including gravity. Tropism is the bending or turning of an organism in response to an external signal such as light or gravity. Plant stems are positively phototropic, meaning they bend toward the light. Plant stems are also negatively gravitropic, meaning they grow upward against the force of gravity. In contrast, plant roots grow down and away from the light. Thus, roots are positively gravitropic and negatively phototropic.
In 1880, Charles Darwin, working with his son Francis, carried out some of the earliest research on phototropism. Observing that grass seedlings bend toward light, Darwin conducted experiments to determine what part of the plant detects light. When he covered just the tip of the young grass plants, they no longer grew toward the light, but when he buried them in fine black sand so that only their tips were exposed, they did. This experiment established the shoot tip as the site where the light was perceived but left open the question of what kind of signal caused the plants to bend (Fig. 31.20).
FIG. 31.20: How do plants grow toward light?
BACKGROUND In 1926, Fritz Went, a Dutch graduate student, followed up on the experiments of Charles and Francis Darwin. In particular, Went sought to understand how the perception of light by the tip of the plant led to a growth response farther down the stem. He reasoned that if the signal from the shoot tip was a chemical, he could isolate it by cutting the tip off of a growing plant and putting it on a block of gelatin. The gelatin would act like a trap, capturing any chemical that moved out of the shoot tip.
HYPOTHESIS A chemical signal links the perception of light by the shoot tip with the growth of the stem toward light.
EXPERIMENT AND RESULTS Went cut off the tips of young oat plants (Avena sativa) and placed them on gelatin blocks for a period of 1 to 4 hours. Removing the tip of the plant caused the plants to stop elongating. When the gelatin block was placed on top of the cut plant (Experiment 1), growth resumed. This experiment demonstrated that a growth-inducing chemical was exported from the tip of the young oat plants.
Interestingly, Went found that if the block of gelatin was not centered on the tip but instead was a bit off center, the plant bent away from the side of the gelatin block (Experiment 2). In this case, the side of the plant exposed to the gelatin block grew faster, causing bending, because it received more of the chemical signal. This experiment demonstrates that increasing the amount of auxin on one side of a stem results in bending.
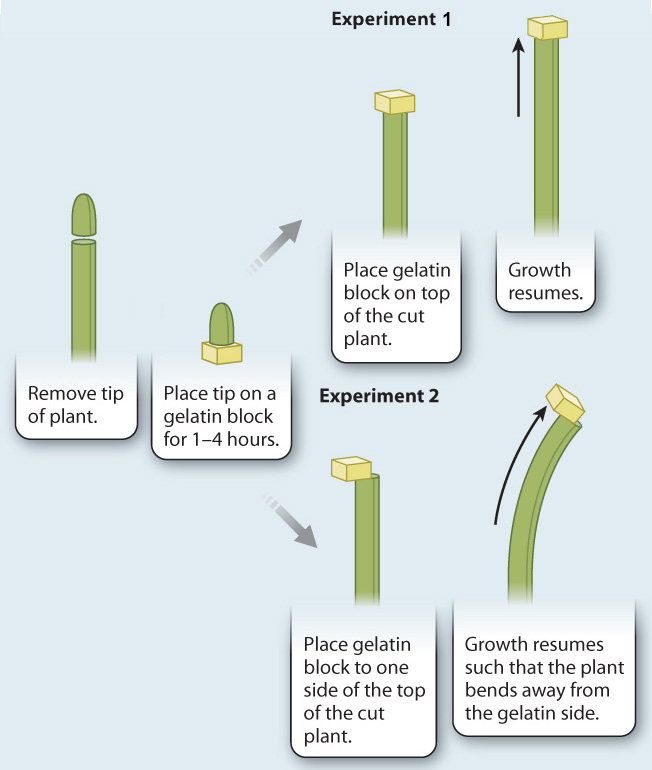
CONCLUSION A signal is produced in the tip of the plant and travels downward through the stem, toward the roots. This signal stimulates growth. Light causes the signal to move to the opposite side, stimulating growth on that side, which in turn causes the plant to bend toward the light. This signal is now known to be a plant hormone, called auxin from the Latin word augere, “to increase.”
FOLLOW-UP WORK Went followed up his experiments by determining how the level of auxin relates to the degree of curvature. This assay became known as the Avena curvature test, after the experimental species. Other experiments showed that auxin moves in a directed fashion by polar transport. Later, auxin was shown to be indole-3-acetic acid (IAA).
SOURCES Darwin, C., and F. Darwin. 1880. The Power of Movement in Plants. London: John Murray; Went, F. W. 1926. “On Growth-Accelerating Substances in the Coleoptile of Avena sativa.” Proceedings of the Royal Academy of Amsterdam 30:10–19.
31-18
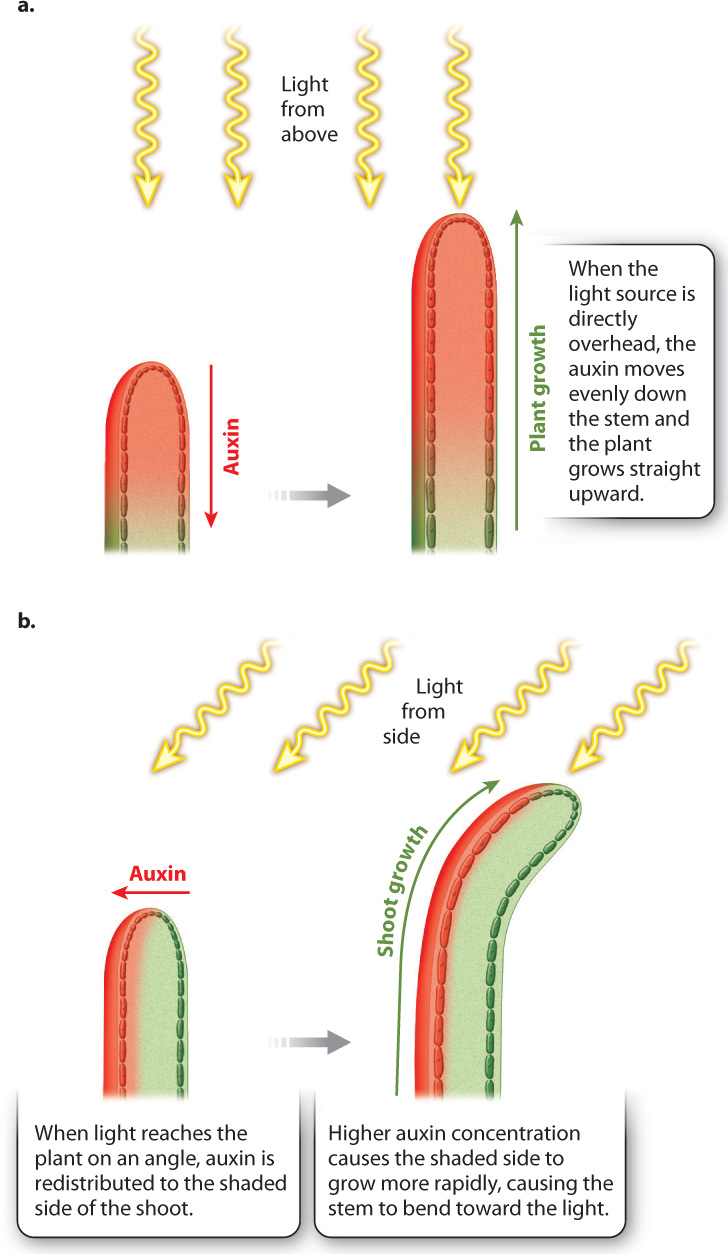
In Chapter 30, we saw that plants produce photoreceptors that allow them to detect and respond to light. If a plant is exposed to light from only one side, only the photoreceptors on the illuminated side are activated. Phototropism in plants involves a photoreceptor that absorbs blue wavelengths of light. The absorption of blue light by the photoreceptor causes auxin to be transported preferentially to the shaded side of the stem. This results in high concentrations of auxin on the shaded side and lower auxin levels on the illuminated side. Because auxin stimulates cell expansion in stems, the shoot grows faster on the shaded side than it does on the illuminated side, causing the plant to bend toward the light (Fig. 31.21).
Phototropism in roots is similar, in that illuminating a root from the side causes auxin to be redistributed toward the shaded side. In this case, auxin is transported to the shaded side within the root cap, then moves upward into the zone of cell elongation. However, higher auxin concentrations decrease the rate of cell elongation in roots, causing roots to bend away from light. The reason that auxin redistribution leads to such a different outcome in roots compared to shoots is that in roots high concentrations of auxin trigger the production of the hormone ethylene, which reduces cell elongation.
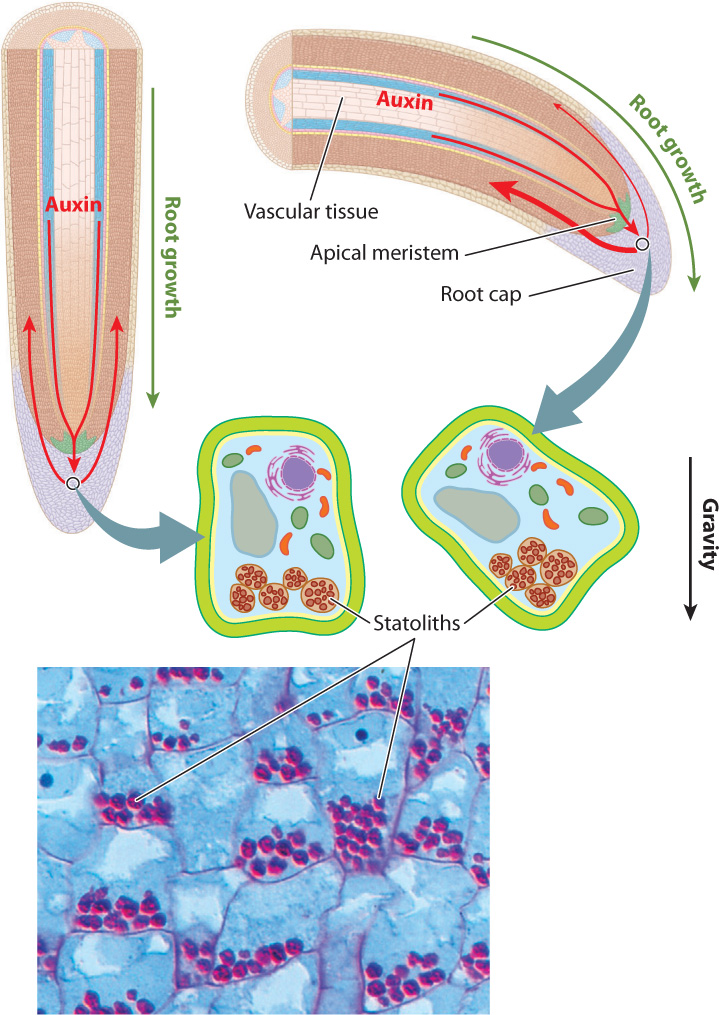
A similar mechanism orients plants with respect to gravity. Moving a plant to a horizontal position results in the transport of auxin to the lower side. The accumulation of auxin on the lower surface causes stems to bend up and roots to turn down. How is the plant able to detect which way is up, which way is down? Recall that plants store carbohydrates as starch. Starch is a large molecule that has a density greater than water. Starch-filled organelles thus sink to the bottom of the cell. The weight of these organelles pressing on the cytoskeleton or membranes is thought to provide plants with information regarding their orientation relative to gravity. In roots, for which gravity is a critical source of information regarding orientation, specialized gravity-sensing cells in the root cap contain large starch-filled organelles known as statoliths (Fig. 31.22). In stems, starch-filled organelles in bundle-sheath cells are thought to play a similar role.
31-19
31.5.2 Plants grow taller and branch less when light levels are low.
A newly germinated seedling must reach the soil surface before its stored resources are used up or it will die. Seeds that germinate in the dark produce completely white seedlings with thin, highly elongated internodes and suppressed leaf development (Fig. 31.23). By putting all their resources into elongation, seedlings grown in the dark maximize their chances of reaching the light. If and when they do, internodal elongation slows markedly and green leaves expand. These dramatic changes are triggered by photoreceptors, which signal the presence of light. The transformation from a dark-grown to a light-exposed seedling involves several photoreceptors, including one that absorbs blue wavelengths and one, phytochrome, that is activated by the absorption of far-red wavelengths.
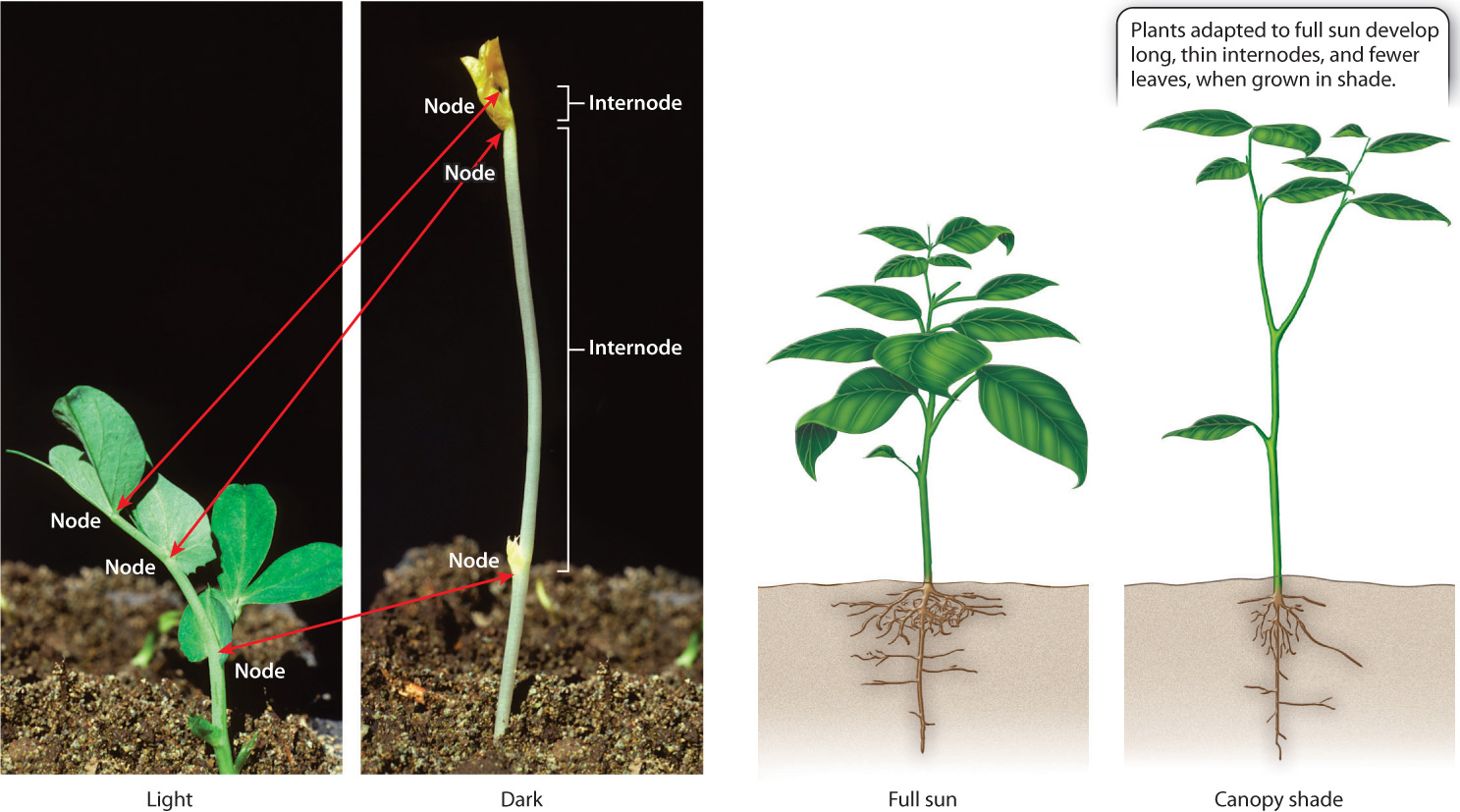
Even after a plant emerges above the soil, the ability to sense and respond to light can enhance its chances for survival. Some species are adapted for shade, producing thin leaves that efficiently use low levels of light; others grow best in direct sunlight. When such sun-adapted species are grown in the shade of another plant, they direct their resources primarily into growing tall. These plant species produce stems that have long, thin internodes and fewer branches, and they invest less in root growth underground.
Interestingly, the effect is much less if the shade is produced by anything other than another plant. The reason for this is that chlorophyll absorbs red wavelengths, but not far-red wavelengths of light. Thus, as light passes through leaves it becomes depleted in red wavelengths. As we saw in Chapter 30, phytochrome is a photoreversible photoreceptor that shuttles between a red-light and a far-red-light absorbing form. It allows plants to detect when they are underneath another plant. It also allows them to sense when they are surrounded by other plants that could grow to shade them in the future because light reflected off their neighbors’ leaves is also depleted in red wavelengths of light. Thus, phytochrome provides an early-warning system of the presence of nearby competitors.
31.5.3 Roots elongate more and branch less when water is scarce.
The developmental sensitivity of plant shoots is mirrored underground. When a plant experiences drought, it produces more roots, and these roots penetrate farther into the soil, in part because they produce fewer lateral branches. By producing deeper roots, a plant increases its chances of reaching moister soil.
The root cap appears to play an important role in allowing roots to sense and respond to the amount of water in the soil. Because the root cap is not well connected to the plant’s vascular system, the water status of its cells provides a good indication of the moisture content of the soil. As soils dry, root cap cells produce a hormone called abscisic acid, commonly abbreviated as ABA (Table 31.1). Abscisic acid stimulates root elongation by suppressing ethylene synthesis. Ethylene affects the growth of cells by influencing the orientation of their cellulose microfibrils. Cells treated with ethylene exhibit an increase in diameter growth and lower rates of elongation. Thus, by inhibiting the production of ethylene, the production of ABA in drought-influenced roots leads to roots that penetrate deeper into the soil.
31-20
31.5.4 Exposure to wind results in shorter and stronger stems.
In the 1980s, scientists studied the genetic model plant Arabidopsis thaliana to learn which genes are expressed in response to different hormones. The experimental treatment involved spraying A. thaliana plants with different solutions containing hormones; control plants were sprayed with water. The treatments were successful in that several genes were strongly up-regulated. However, the same genes were also turned on in the plants sprayed with water. Further experiments showed that what the plants were responding to was neither the hormones nor the water, but the fact that their stems were bent back and forth when the plants were sprayed. This led to the discovery of touch-sensitive genes in plants, which are activated by mechanical perturbation.
This discovery confirmed what foresters and horticulturists had long known. Plants exposed to wind produce stems that are shorter and wider than ones grown in more protected sites. In fact, commercial greenhouses often install fans, in part so that their plants will produce stems robust enough that the plants can thrive when moved outdoors. Flexing a stem back and forth triggers an increase in the synthesis of ethylene. Cells exposed to ethylene expand more in diameter and less in length, resulting in shorter and thicker stems.
31.5.5 Plants use day length as a cue to prepare for winter.
In Chapter 30, we saw how plants can use day length to control when flowers are produced. Not surprisingly, day length also triggers other developmental events, particularly ones that allow plants that persist for more than a year to prepare for winter. One such developmental change is the formation of storage organs, for example in roots. Carbohydrates that accumulate in these structures can support the growth of new leaves and stems the following spring.
A second developmental change in response to day length is the formation of overwintering buds. As the days begin to shorten, plants stop producing photosynthetic leaves and begin forming bud scales (see Fig. 31.6a). Bud scales surround and protect the meristem from ice and water loss. The formation of bud scales accompanies a series of metabolic changes that allow meristems to remain in a dormant state throughout the winter. For example, plugs are produced that block the plasmodesmata between the meristem and the rest of the plant. These plugs prevent any growth-stimulating compounds from reaching the meristem.
The hormone abscisic acid contributes to the formation of overwintering buds, similarly to its role in promoting seed dormancy. Many temperate trees must experience sustained exposure to cold temperatures before they will break dormancy, thus preventing a warm period in the autumn from inappropriately triggering growth. In the spring, lengthening days and warmer temperatures reverse the metabolic changes that accompany dormancy and allow growth to resume.
This section has focused on the environmental context of plant growth and development—how plants alter their development to enhance the uptake of water, nutrients, and sunlight, and to cope with physical stresses such as winter. In the next chapter, we focus on the biological context of plant growth and development—how plants defend themselves from being eaten or parasitized.
31-21