32.1 PROTECTION AGAINST PATHOGENS
In September 1845, potato plants across Ireland began to die. Their leaves turned black and the entire plant began to rot. When the potatoes were dug up, those that were not already rotten soon shriveled and decayed into foul-smelling and inedible mush. The biological agent responsible for the failure of the potato crop looked like a fungus, but in fact was an oomycete called Phytophthora infestans, a type of protist (Chapter 27).
32-2
P. infestans is one example of the diverse pathogens that infect plants. Plant pathogens include viruses, bacteria, fungi, nematode worms, and even other plants, but what they all have in common is the ability to grow on and in the tissues of plants, extracting resources to support their own growth and causing an array of disease symptoms. The outcome for the plant is often death (Fig. 32.1). The fact that most plants appear disease free is testimony to their ability to defend themselves. Plants have an innate immune system that allows them to recognize and respond to pathogens that would otherwise exploit them. In the Irish potato famine, which lasted from 1845–52, these defenses were breached, allowing Phytophthora infestans to spread and grow with devastating effects on the potato crop and, in consequence, the people of Ireland.

32.1.1 Plant pathogens infect and exploit host plants by a variety of mechanisms.
The epidermis, with its thick walls and waxy cuticle, is a plant’s first line of defense against pathogens. Viruses and bacteria cannot penetrate the cuticle and so commonly enter through wounds or on the piercing mouthparts of insects and nematodes. Stomata form a natural entry point that enables bacteria, oomycetes, and fungi to gain access to leaves (Fig. 32.2). Some bacteria and fungi even secrete chemicals that prevent stomata from closing, improving their ability to infect plant tissues. Other pathogens, notably parasitic plants and some species of fungi, secrete enzymes that weaken epidermal cell walls. These invaders then force their way through the weakened epidermis and gain access to the plant interior. Mechanical cultivation can damage crop plants, accelerating the spread of pathogens.
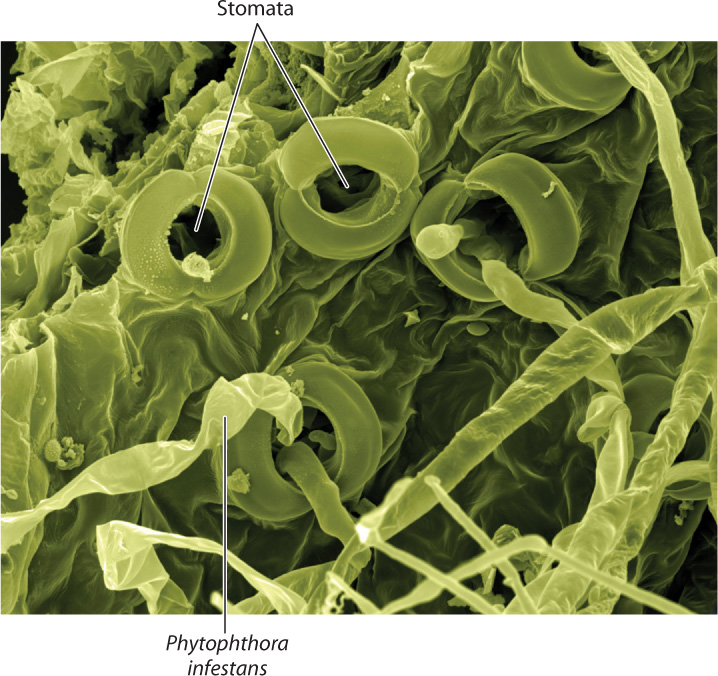
Once inside the plant, fungi can spread by growing through plant cell walls. Viruses move from one plant cell to another through plasmodesmata. Because the genomes of most plant viruses are single strands of RNA, they can travel by the plant’s natural mechanism for moving messenger RNA between cells. The plant vascular system provides a highway that allows pathogens to move over longer distances. Viruses are transported within the phloem, whereas bacteria and fungi move through the xylem. During the 1950s, commercial banana production in Mesoamerica was nearly wiped out by Fusarium oxysporum, a fungal pathogen whose spores are carried in the xylem. More recently, the California wine industry has been threatened by Pierce’s disease, which results from infection by Xylella fastidiosa, a bacterium that also moves through the xylem.
Biotrophic pathogens obtain resources from living cells, whereas necrotrophic pathogens kill cells before colonizing them. Defenses effective against one type are not necessarily effective against the other. Thus, plants respond differently to these two classes of pathogens. Viruses are biotrophic pathogens; they must hijack a living cell’s machinery to reproduce. Bacteria and fungal pathogens are present in both groups. For example, wheat leaf rust (Puccinia species) is a biotrophic fungus that significantly reduces wheat yields (Chapter 34). Rice blast (Magnaporthe grisea) is a necrotrophic fungus that each year reduces rice production by an amount that would feed 60 million people.
32-3
Parasitic plants obtain resources by infecting other plants (Fig. 32.3). Parasitic plants produce structures that penetrate into the stems or roots of other plants, eventually tapping into the host plant’s vascular system. Some, like some mistletoes, are xylem parasites: They obtain water and nutrients from the xylem of the host plant without expending the costs of producing roots themselves. Other plant pathogens target both the xylem and the phloem. In the most extreme cases, the parasite receives all its carbohydrates from its host and carries out no photosynthesis of its own. Rafflesia, famous for producing the largest flower in the world, grows entirely within the tissues of its host, emerging only when it produces its giant flowers. The ability to parasitize other plants has evolved independently multiple times. Currently, more than 4000 species of parasitic plants have been identified.
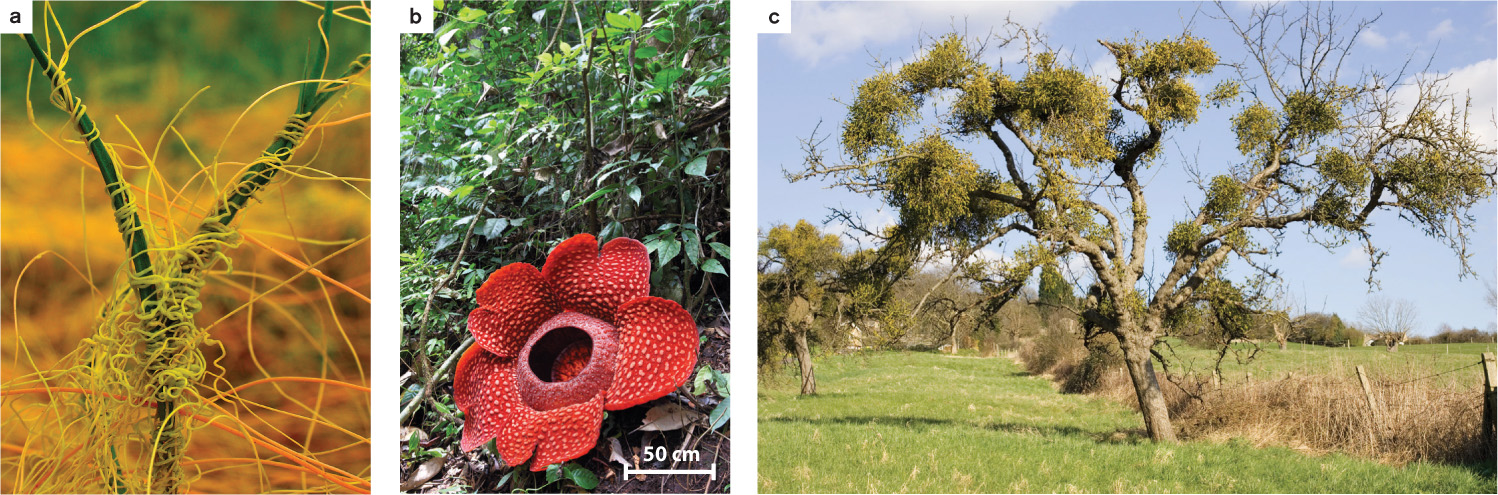
How do parasitic plants find an appropriate host? Mistletoe fruits, which are eaten and dispersed by birds, have a sticky layer that adheres to the stems and branches of trees that the plant will parasitize. One of the most destructive plant pathogens is witchweed (Striga species), which infects the roots of a number of crop species, including corn, sorghum, and sugarcane. Striga seeds remain dormant in the soil until they detect chemicals released from the roots of suitable host species. The chemicals that trigger the germination of Striga seeds are strigolactones (Chapter 31), a plant hormone also involved in establishing symbiotic interactions between plant roots and mychorrhizal fungi (Chapter 29) and inhibiting the outgrowth of axillary buds. Striga infects more than 40% of all agricultural areas in Africa, driving down crop yields, in some cases to zero.
32.1.2 An innate immune system allows plants to detect and respond to pathogens.
Most plants are at risk of infection by only a small subset of the many plant pathogens known. Host plants of a particular pathogen are the species that can be infected by that pathogen. Not all infections have a significant effect on the host plant, however. Virulent pathogens are able to overcome the host plant’s defenses and lead to disease. In contrast, avirulent pathogens damage only a small part of the plant because the host plant is able to contain the infection. Whether an interaction is virulent or avirulent depends on the genotypes of both the pathogen and its host plant.
In your own body, an innate immune system provides a generalized first line of defense against pathogens (Chapter 43). Plants, too, have an innate immune system that allows them to detect pathogens and mount an appropriate response. A key feature of the immune system is that plants, like animals, can recognize a cell or virus as foreign. How do they accomplish this?
Plant cells have protein receptors (Chapter 9) that bind to molecules produced by pathogens and recognize them as foreign to the plant body. Binding changes the conformation of the receptor, triggering a cascade of reactions that enhance the plant’s ability to resist infection.
32-4
The plant immune system has two parts, one general, or basal, and the other specific (Fig. 32.4). The basal branch of the plant immune system consists of receptors located on the plasma membrane (Fig 32.4a). These receptors recognize highly conserved molecules generated by broad classes of pathogens. Examples of these molecules are flagellin, present in the flagella of bacteria, and chitin, a component of the fungal cell wall. When one of these molecules binds to the plant’s receptor, an array of defense mechanisms are triggered that help protect the plant from infection.
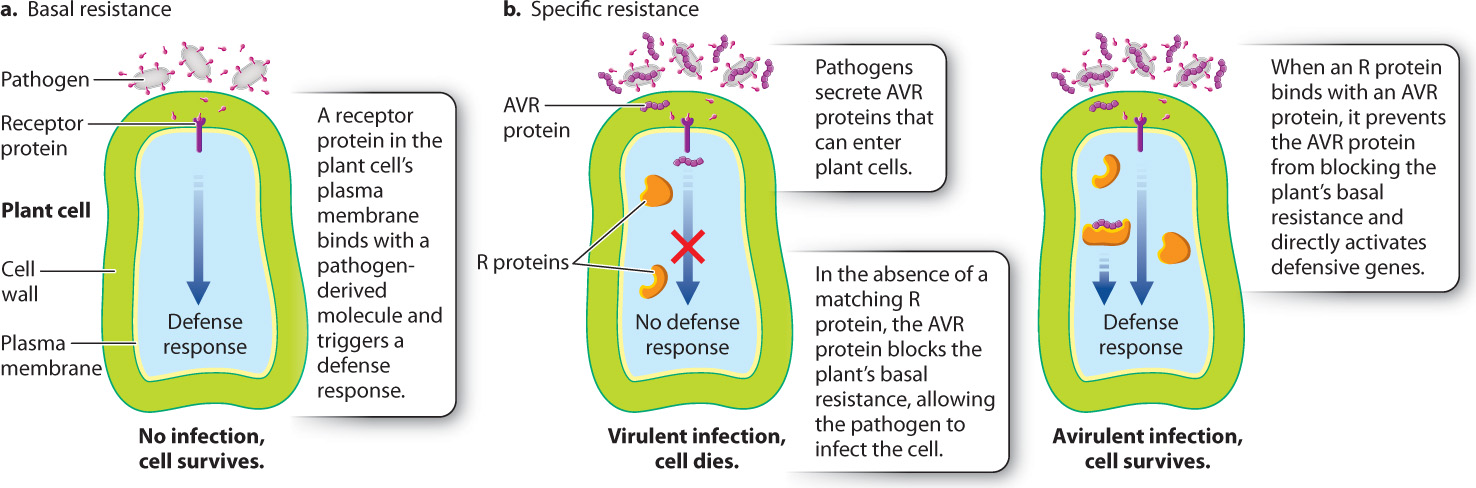
The second branch of the plant immune system allows plants to resist specific pathogens (Fig. 32.4b). It also consists of receptors, in this case located inside the cell rather than on the plasma membrane. Plants harbor hundreds of these receptors, called R proteins, each expressed by a different gene —these genes are collectively called R genes (the “R” stands for “resistance”). Pathogens produce proteins called AVR proteins that enter into plant cells and facilitate infection (“AVR” stands for “avirulence”). Some AVR proteins block the basal branch of the immune system, while others cause the cell to secrete molecules that the pathogen needs to support its own growth. Each R protein recognizes a specific AVR protein. When an R protein binds with a pathogen-derived AVR protein, it both prevents the AVR protein from blocking the plant’s defenses and activates additional defenses. Because this branch of the immune system depends on interactions between specific plant and pathogen genes, it is commonly called the gene-for-gene model of plant immunity.
The large number of R genes is the result of an evolutionary arms race that occurs as pathogens evolve ways to evade their host’s detection systems. Pathogens improve their success rate when mutation results in an AVR protein that does not bind with any of the R proteins produced by its target. For example, P. infestans, the pathogen responsible for the Irish potato famine, produces many more AVR proteins than related Phytophthora species. The large number of AVR proteins found in P. infestans increases the probability that at least one of these proteins is not detected by the infected plant. Conversely, when pathogens are successful, natural selection favors plant populations that produce new R proteins capable of binding to the AVR proteins of the invader.
Question Quick Check 1
UuLgs+wJ6Ksi9ETXXiFee3853VTQGSXYrELn3udX+24hOWPLqOQm9U3K6ytWoy5QZ/MVvQ2OOna7ZxOXjwOcviOKynMAJ3xNk+ofC7KsedGPpD/WYKwVO58Q7yPWjJ4Qiy69rGvs5wt/vRU7vpZjqxLbNJptyoTQjDjR+fPw8F9c0MdSMmnTQlGxZrw=32.1.3 Plants respond to infections by isolating infected regions.

Once a plant has detected a pathogen, how does it protect itself? One way is by reinforcing its natural barriers against pathogens. For example, plants commonly defend themselves by strengthening their cell walls, closing their stomata, and plugging their xylem. Or the plant can counterattack. Plants produce a variety of antimicrobial compounds, including ones that attack bacterial or fungal cell walls. A third type of defense is known as the hypersensitive response. In this case, uninfected cells surrounding the site of infection rapidly produce large numbers of reactive oxygen species, which trigger cell wall reinforcement and cause the cells to die (Fig. 32.5). The death of surrounding cells create a barrier of dead tissue that prevents the spread of biotrophic pathogens and slows the growth of necrotrophic ones.
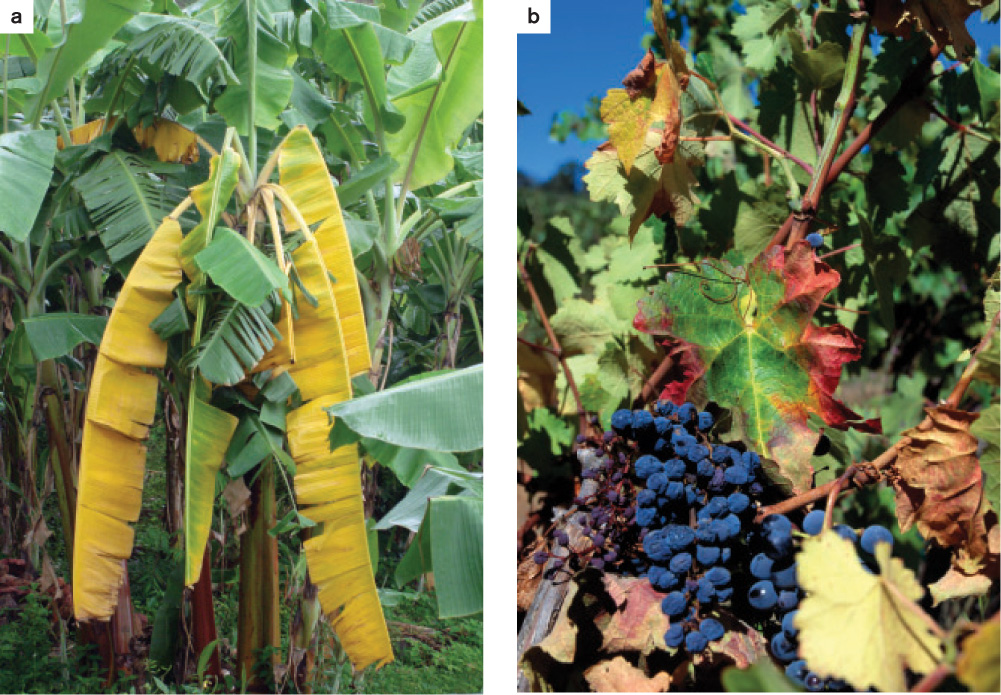
Plants can defeat pathogens by isolating infected regions to a far greater degree than is possible in most animals. For example, plants respond to the presence of xylem-transported pathogens by plugging xylem conduits. If the infection is caught in time, the pathogen is prevented from spreading throughout the plant. But if containment fails and the pathogen spreads, the plant may contribute to its own demise by blocking its entire vascular system. Virulent pathogens that move through the xylem cause a variety of disorders collectively referred to as vascular wilt diseases (Fig. 32.6). The widespread death of native elm trees in both Europe and North America is the result of a vascular wilt disease caused by a fungus introduced from Asia, but first identified in the Netherlands and therefore referred to as Dutch elm disease.
32-5
32.1.4 Mobile signals trigger defenses in uninfected tissues.
In 1961, American plant pathologist A. F. Ross reported that when individual tobacco leaves were infected with the tobacco mosaic virus (TMV), they developed necrotic patches characteristic of a hypersensitive response. However, when uninfected leaves of the same plant were subsequently exposed to the virus, they suffered little or no damage (Fig. 32.7). Ross called this ability to resist future infections systemic acquired resistance (SAR). Initially reported for viral infections, SAR occurs in response to a wide range of pathogens, although only when infection results in necrosis due to either a hypersensitive response or a necrotrophic pathogen.
FIG. 32.7: Can plants develop immunity to specific pathogens?
BACKGROUND Tobacco is susceptible to an infectious disease that causes the leaves to turn a mottled yellow, hence the name of the disease—tobacco mosaic disease. In the 1890s, experiments with filters showed that the infectious agent was smaller than a bacterium, a finding that led to the discovery of viruses. Some plants succumb to the disease, but others do not. Are the plants that survive the initial infection immune from further attacks?
HYPOTHESIS Plants that survive infection with tobacco mosaic virus (TMV) have acquired immunity and therefore resist further attack.
EXPERIMENT American plant pathologist A. F. Ross infected one leaf on a tobacco plant with TMV. One week later, he exposed another leaf on the same plant to the virus.
RESULTS Plants that had been previously exposed to TMV showed no signs of infection when exposed to the virus a second time.
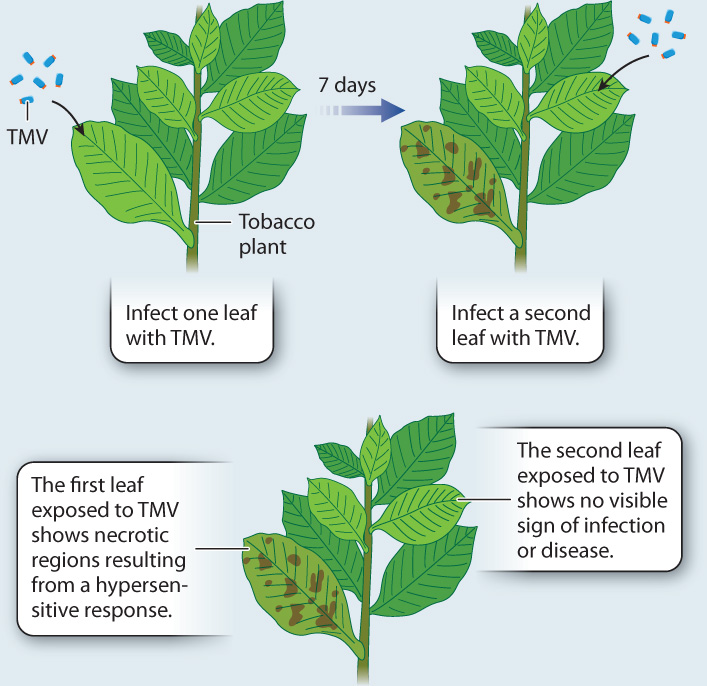
CONCLUSION Tobacco leaves that have never been exposed to TMV can acquire resistance to the pathogen if another leaf on the same plant has been previously exposed. This result indicates that a signal has been transmitted from the originally infected leaf to the undamaged parts of the plant, and that the transmitted signal subsequently triggers the development of an immune response that protects the plant from further infection.
FOLLOW-UP WORK Plants are now known to acquire immunity to a wide range of pathogens in addition to viruses. The genetic basis for the immune response is a system in which proteins encoded by plant R genes recognize specific AVR proteins produced by pathogens.
SOURCE Ross, A. F. 1961. “Systemic Acquired Resistance Induced by Localized Virus Infections in Plants.” Virology 14:340–358.
How do uninfected leaves acquire resistance to pathogens? One hypothesis is that a chemical signal is transported from the infected region through the phloem. This chemical signal then triggers the expression of genes encoding many of the same proteins that defend against the pathogen in infected cells. The identity of the mobile signal has proved elusive, but experiments show that salicylic acid—the chemical basis of aspirin—is required for SAR. In experiments in which salicylic acid accumulation was blocked, plants exposed to pathogens failed to develop SAR. Furthermore, the experimental exposure of plants to salicylic acid induces SAR. The methylated form of salicylic acid, known familiarly as oil of wintergreen, is also a potent inducer of defense responses. Methyl salicylic acid is volatile and vaporizes readily, giving rise to the characteristic wintergreen smell. It has been proposed that the transport of methyl salicylic acid vapor through the air may play a role in signaling the presence of pathogens to more distant regions of the plant.
32-6
Systemic acquired resistance increases the ability of uninfected tissues to resist infection. In general, these responses are not highly specific. Rather, SAR activates defenses effective against broad classes of pathogens. Only in the case of viral infections, discussed next, is the response targeted to an individual pathogen.
Question Quick Check 2
ygkz0TV9+HfzL4EMrUabN8r0c1NaC/ny9SvLXDIxcIri9vyu5F4AzPyZ5ApVICuU15y5jijORHzHsAftBi+fmUa5SDH+nAYoy20ZJAjhAtZzfHdr/9joodwqLMN64D2532.1.5 Plants defend against viral infections by producing siRNA.
Viruses can be potent infectious agents, but plants have evolved responses to viral infection—including a hypersensitive response—that are much like those mounted against other pathogens. In addition, plants have a form of defense that targets the virus itself (Fig. 32.8). Most plant viruses have genomes made of single-stranded RNA (ssRNA). During the replication of the viral genome, double-stranded RNA molecules (dsRNA) are formed. The production of double-stranded viral RNA opens the viral genome to counterattack by its host plant. Because plant cells do not normally make double stranded RNA, the replicating viral genomes are identified as foreign. Enzymes produced by the plant cell cleave the double-stranded RNA molecules into small pieces of 21 to 24 nucleotides, forming fragments called small interfering RNA, or siRNA (Chapter 3). These fragments play a role in targeting and destroying single-stranded RNA molecules that have a complementary sequence—that is, the viral genome (Chapter 19). The virus is unable to replicate and thus cannot spread. In this way, the plant cell uses RNA chemistry to eliminate viral infections.
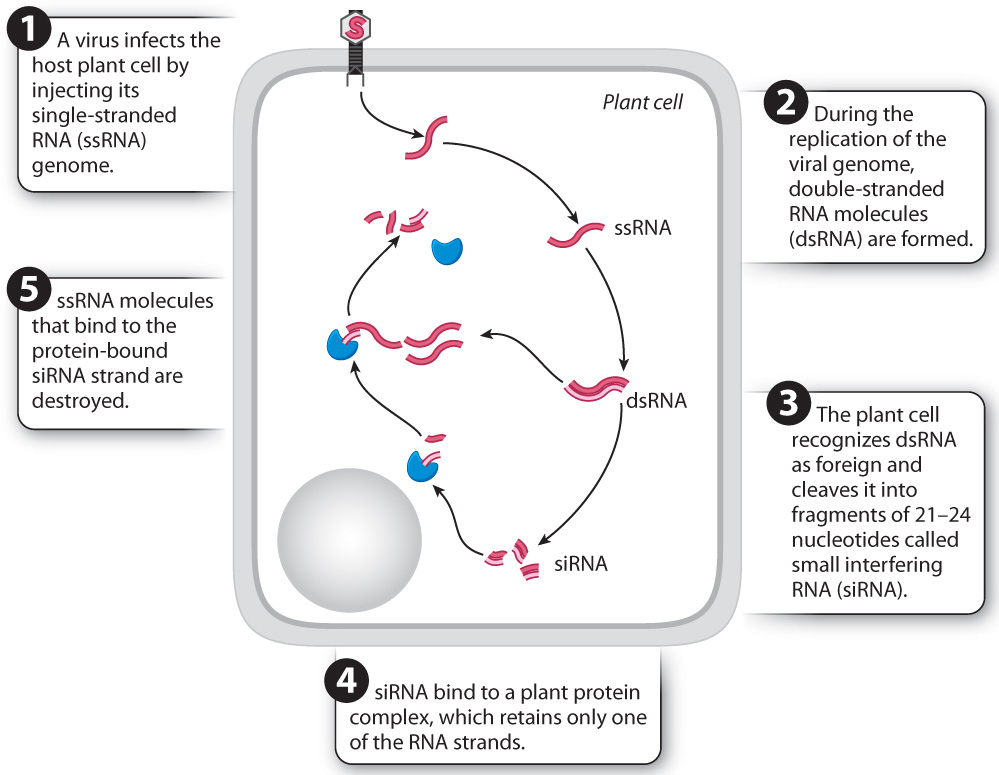
When a cell is attacked by a virus, the siRNA molecules that are produced in response to the initial infection can also spread, allowing the plant to acquire immunity against specific viruses. The siRNA molecules move through plasmodesmata, enter the phloem, and from there spread throughout the plant. Thus, the systemic response to viral infection involves both general defense responses mediated by salicylic acid and the transport of highly specific molecules that can target and destroy viral genomes throughout the plant.
32-7
32.1.6 A pathogenic bacterium provides a way to modify plant genomes.
Some pathogenic bacteria alter their host plant’s biology by inserting their own genes into the host’s genome. One such pathogen, Rhizobium radiobacter (formerly known as Agrobacterium tumifaciens), is beneficial to humans, despite the fact that it can cause widespread damage to crops such as apples and grapes. The reason? This bacterium’s naturally evolved ability to incorporate its DNA into the genome of its host plant provides a surprisingly easy way for biotechnologists to engineer plants.
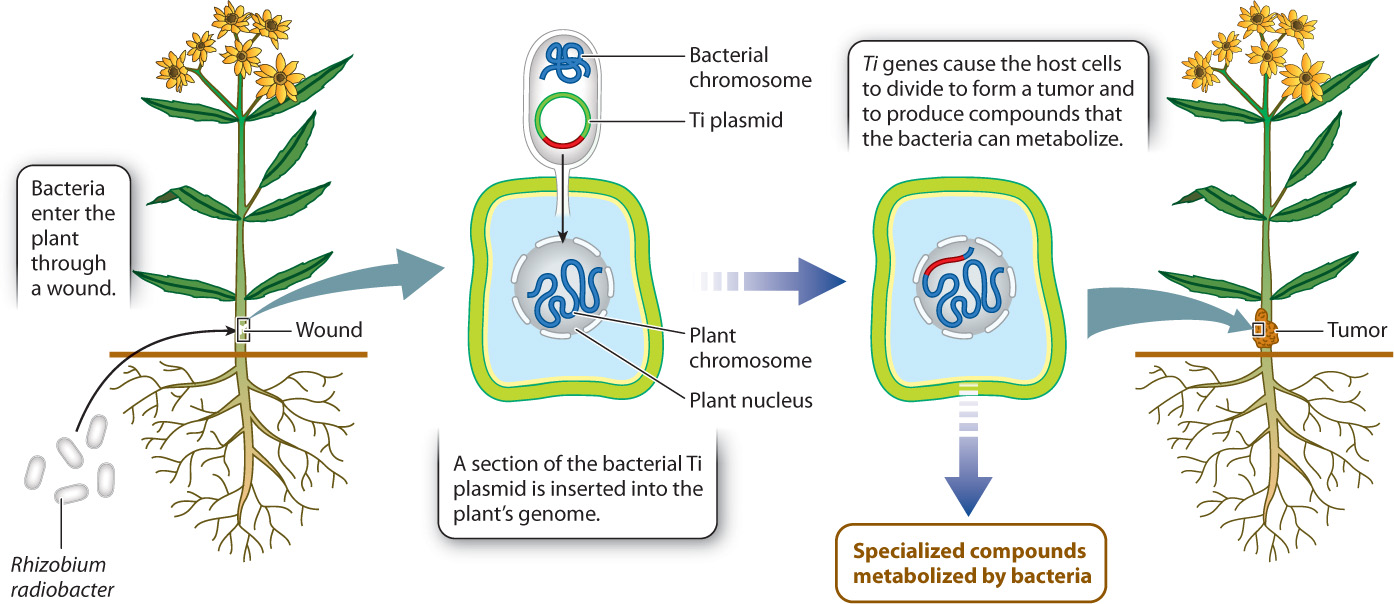
R. radiobacter is a soil bacterium with virulent strains that infect the roots and stems of many plants. Virulent bacteria enter plants through wounds and move through the plant’s cell walls, propelled by flagella. A tumor, or gall, forms at the point of infection, often where the stem emerges from the soil, and for this reason R. radiobacter infection is called crown gall disease. R. radiobacter alters the growth and metabolism of infected cells by inserting some of its own genes into the chromosomal DNA of the host plant (Fig. 32.9).
32-8
Virulent strains of R. radiobacter contain a plasmid, called the Ti plasmid, which is a small (∼200 kb) circular DNA molecule containing the genes that will be integrated into the host cell’s genome, as well as all the genes needed to make this transfer. Some of the transferred genes—including ones that result in the synthesis of the two plant hormones auxin and cytokinin (Chapter 31)—cause infected cells to proliferate and form a gall. Other transferred genes induce cells in the gall to produce specialized compounds that the bacteria use as sources of carbon and nitrogen for growth. Note that because plant cells do not move (in contrast to animal cells), there is no possibility that the tumor-forming cells will spread to other parts of the plant.
Biologists interested in improving crops and other plants make use of R. radiobacter’s ability to insert genes into the host genome. The first step is to replace the genes in the Ti plasmid that will be inserted into the plant genome. Genes for gall formation are removed, and genes of interest are inserted. Genes of interest include those whose products increase the plant’s nutritional value or confer resistance against disease. Researchers need some way to identify which cells have taken up the genes, and so a marker gene, for example one conferring antibiotic resistance, is typically included. In this way, cells that survive an application of the antibiotic are known to have successfully incorporated the Ti plasmid into their genome.