33.3 SPORE-DISPERSING VASCULAR PLANTS
As noted in Chapters 29 and 30, vascular plants differ from bryophytes in several key aspects of form and life cycles. In vascular plants the diploid sporophyte is larger than the gametophyte and becomes capable of supplying all of its own needs. The sporophyte achieves greater height and independence with the help of vascular tissues that pull water from the soil and distribute carbohydrates throughout the plant body.
Vascular plants can be divided into two groups according to how they complete their life cycle. Lycophytes, as well as ferns and horsetails, disperse by spores and rely on swimming sperm for fertilization, just as occurs in bryophytes. Gymnosperms and angiosperms are seed plants: They are dispersed through the movement of seeds and fertilized through the movement of pollen. In this section, we focus on the spore-dispersing vascular plants: lycophytes, and ferns and horsetails. In both groups, the sporophyte dominates in terms of physical size; the gametophyte is only a few centimeters, thalloid in structure, and typically tolerant of desiccation. Thus, our discussion emphasizes the sporophytes of these plants. However, it is important to remember that because their gametophytes release sperm into the environment, they, like the bryophytes, are dependent on surface moisture for fertilization.
33.3.1 Rhynie cherts provide a window into the early evolution of vascular plants.
Fossils recovered from a single site in Scotland document key stages in the evolution of vascular plants. Four hundred million years ago, near the present-day village of Rhynie, a wetland community thrived in a volcanic landscape. Mineral-laden springs similar to those found today in Yellowstone Park encased the newly deposited remains of local populations in chert, a mineral made of silica (SiO2). These fossils preserve plants, fungi, protists, and small animals in remarkable detail. Rhynie cherts provide our best view of early vascular plants, which were small (up to about 15 cm tall) photosynthetic structures that branched repeatedly, forming sporangia at the tips of short side branches (Fig. 33.6). These plants had no leaves, and their only rooting structures were small hairlike extensions on the lower parts of stems that ran along the ground. The stems had a cuticle with stomata, and a tiny cylinder of vascular tissue ran through the center. Many of the gametophytes also had erect cylindrical stems, not at all like the reduced gametophytes of living vascular plants.
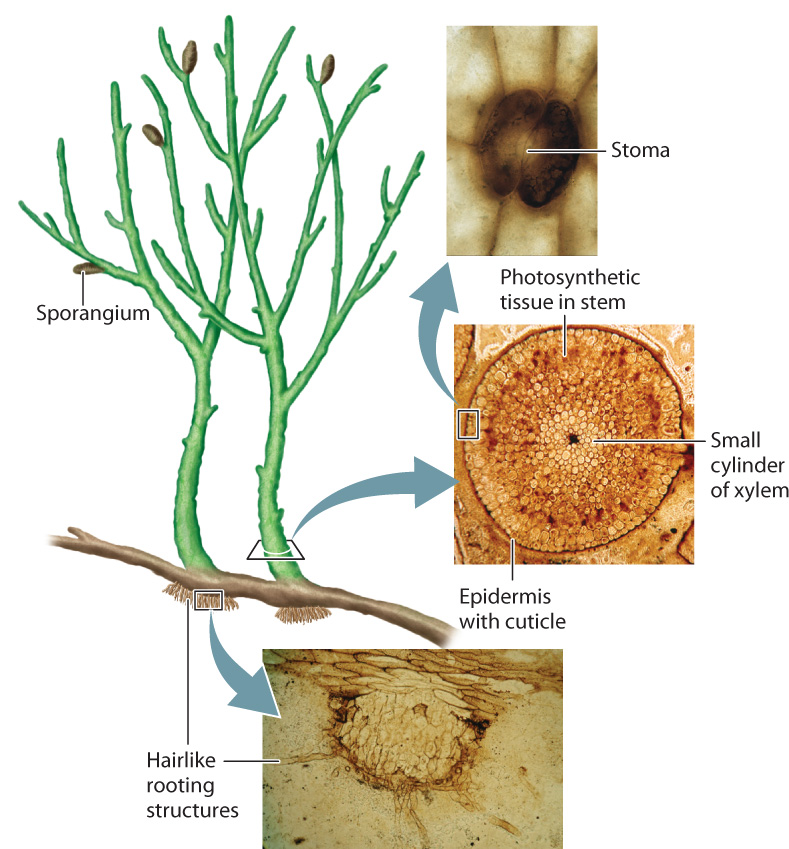
Rhynie cherts preserve another important glimpse of early plant evolution: Some fossils have a branched sporophyte, but no evidence of vascular tissue. Evidently, plants evolved the upright stature we associate with living vascular plants before they evolved the differentiated tissues of the vascular system (xylem and phloem). Once vascular tissues had evolved, however, they played a key role in shaping subsequent evolution of the plant sporophyte.
33.3.2 Lycophytes are the sister group of all other vascular plants.
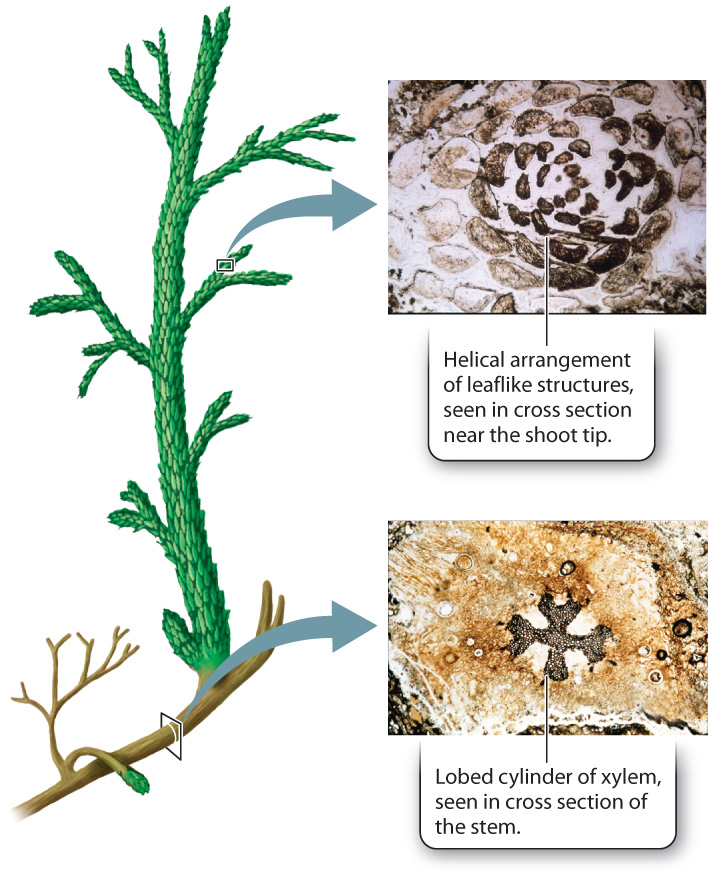
One of the fossil populations in Rhynie cherts is notably larger and more complex than the others. These fossils show leaf-like structures arranged on the stem in a spiral pattern. Through the stem runs a thick-lobed cylinder of xylem (Fig. 33.7). These are among the earliest fossils of lycophytes, an early-branching group of vascular plants that can still be found from tropical rain forests to Arctic tundra. Because lycophytes are the earliest branching group of vascular plants, they are the sister group to all other vascular plants (see Fig. 33.1).
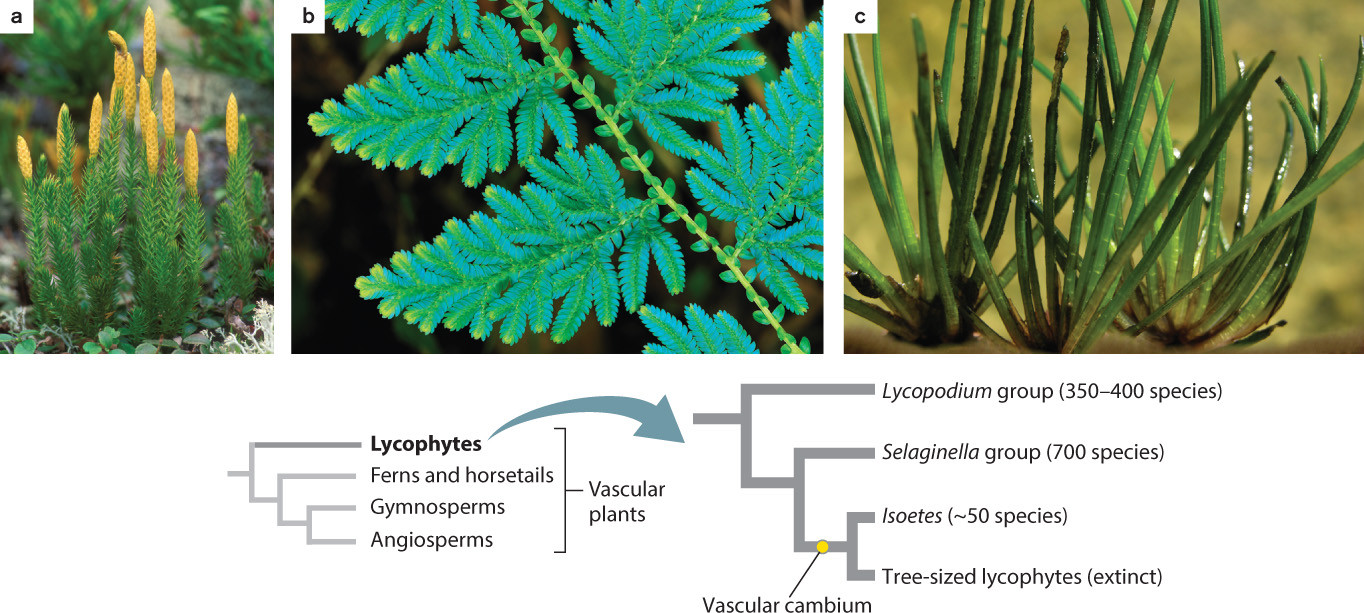
Fossils indicate that the leaves and roots of lycophytes evolved independently from those of ferns, horsetails, and seed plants. A distinctive feature of lycophyte leaves is that they have only a single vein, in contrast to the more complex leaf venation found in other vascular plants. About 1200 species of lycophytes can be found today. Plants assigned to the genus Lycopodium and its close relatives (Fig. 33.8a) grow low to the ground and produce branching stems covered by small leaves arranged in a helical pattern. Roots are produced from stems growing along the ground, while spores form in sporangia borne on leaves, either those arranged along the stems or those clustered near stem tips.
A second group of lycophytes, called Selaginella, have leaves that may be helically arranged around the stem or occur in four distinct rows with the leaves held in a single plane, giving these branched stems a distinctive appearance (Fig. 33.8b). Many species of Selaginella live as epiphytes in tropical forests. A few are vines and some, called resurrection plants, live in seasonally dry habitats where they tolerate desiccation, as more commonly do bryophytes.
The most distinctive living lycophytes are the quillworts (genus Isoetes), which are small plants that live along the margins of lakes and slow moving streams (Fig. 33.8c). A few species are desiccation tolerant and so can live in ponds that dry up in summer. Uniquely among living lycophytes, they have a vascular cambium. Seeing a quillwort today, you would never guess that its relatives once included enormous trees.
33.3.3 Ancient lycophytes included giant trees that dominated coal swamps about 320 million years ago.
Fossils show that ancient lycophytes evolved additional features convergently with seed plants, including a vascular cambium and cork cambium that enabled them to form trees up to 40 m tall and, in some cases, reproductive structures similar to seeds (Fig. 33.9). Indeed, swamps that formed widely about 320 million years ago were dominated by tree-sized lycophytes. Unlike the vascular cambium of seed plants, the vascular cambium of these lycophytes (Fig. 33.10) produced relatively little secondary xylem and no secondary phloem. Instead, the giant lycophytes relied on thick bark for mechanical support. Thus, tree-sized lycophytes were markedly different from the trees familiar to us today.
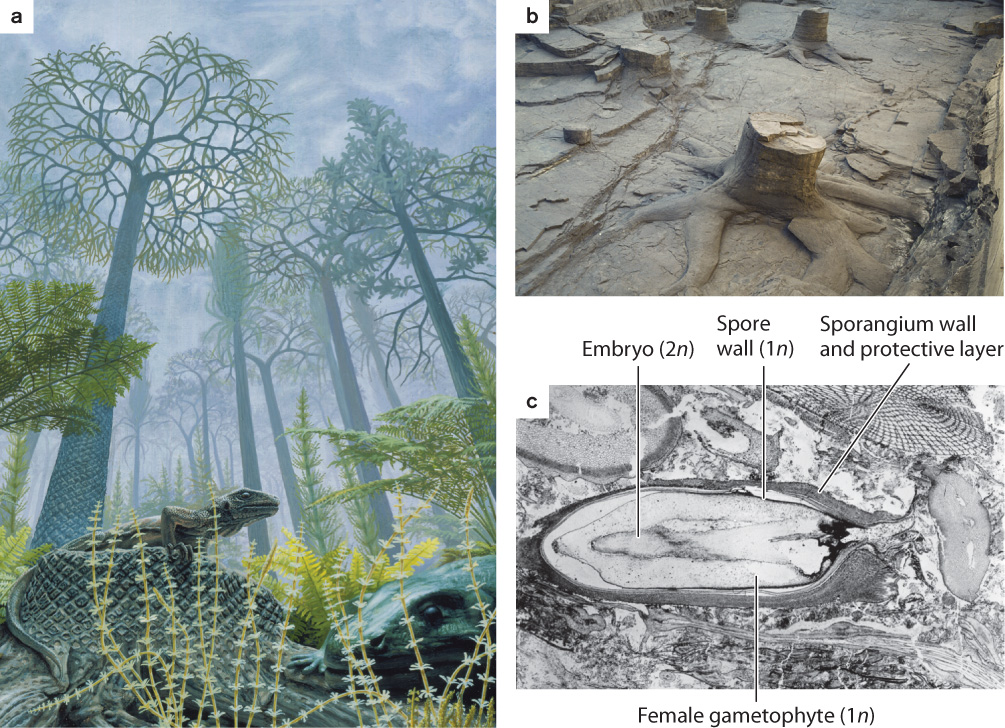
FIG. 33.10: Did woody plants evolve more than once?
OBSERVATIONS Today, the vascular cambium occurs almost exclusively among seed plants, with only limited development of secondary xylem in some adder’s tongue ferns and quillwort lycophytes (Isoetes species). The fossil record, however, shows that other plants, now extinct, also had a vascular cambium.
HYPOTHESIS The vascular cambium evolved convergently in several different groups of vascular plants.
EXPERIMENT AND RESULTS The vascular cambium is recorded in fossils by xylem cells in rows oriented radially in the stem or root. Thus, the giant tree lycophytes of Carboniferous coal swamps (the top photo at the lower right) had a vascular cambium, as did extinct tree-sized relatives of the horsetails (the bottom photo) and other extinct horsetail relatives. Phylogenetic trees generated from morphological features preserved in fossils unambiguously show that woody lycophytes, woody horsetail relatives, and the group of seed plants and progymnosperms did not share a common ancestor that had a vascular cambium. Moreover, anatomical shows that the vascular cambium of extinct woody lycophytes and the giant horsetails Archaecalamites and Calamites generated secondary xylem but not secondary phloem, unlike the vascular cambium of seed plants. Living horsetails do not have a vascular cambium, but fossils show that they are descended from ancestors that did make secondary xylem and have lost that trait through evolution.
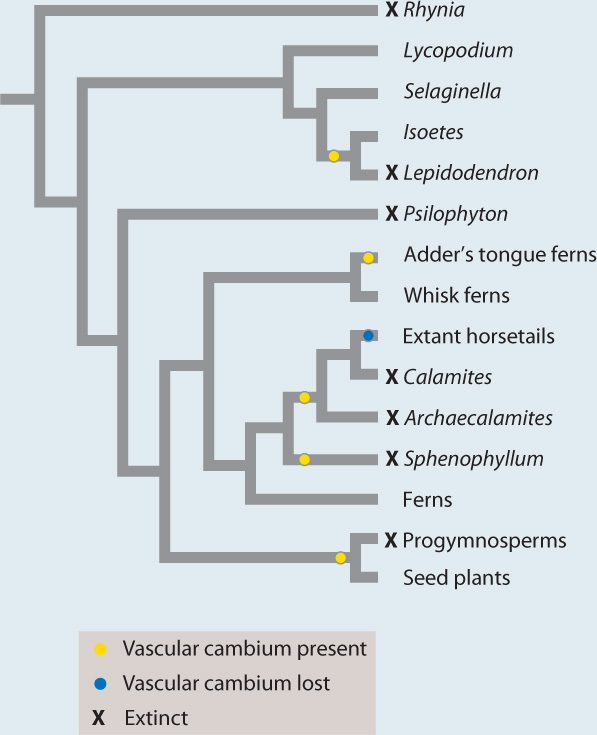
CONCLUSION Fossils and phylogeny support the hypothesis that the vascular cambium and, hence, wood evolved more than once, reflecting a strong and persistent selection for tall sporophytes among vascular plants.
SOURCE Taylor, T. N., E. L. Taylor, and M. Krings. 2009. Paleobotany. Amsterdam: Elsevier.
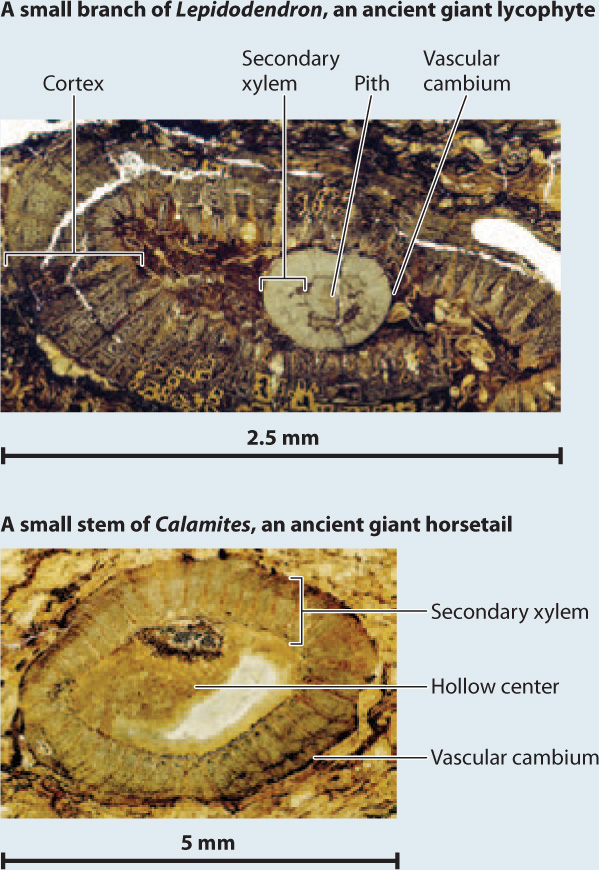
These forest giants were not outcompeted by seed plants, but persisted for millions of years in swampy environments alongside early seed plants. It was environmental change that spelled their doom. When changing climates dried out the swamps, the large lycophytes disappeared, along with their habitat. Little Isoetes was left as the only living reminder of a once-dominant group of plants. However, the other legacy of these ancient forests is the coal deposits we mine today. As trees died and fell over into the swamp, their bodies decomposed slowly. Over time, as material accumulated and became buried, the combined action of high temperature and pressure converted the dead organic matter first into peat and then into the carbon-rich material we call coal. Thus, a major source of energy used today by humans derives originally from photosynthesis carried out by these lycophyte trees.
33.3.4 Ferns and horsetails are morphologically and ecologically diverse.
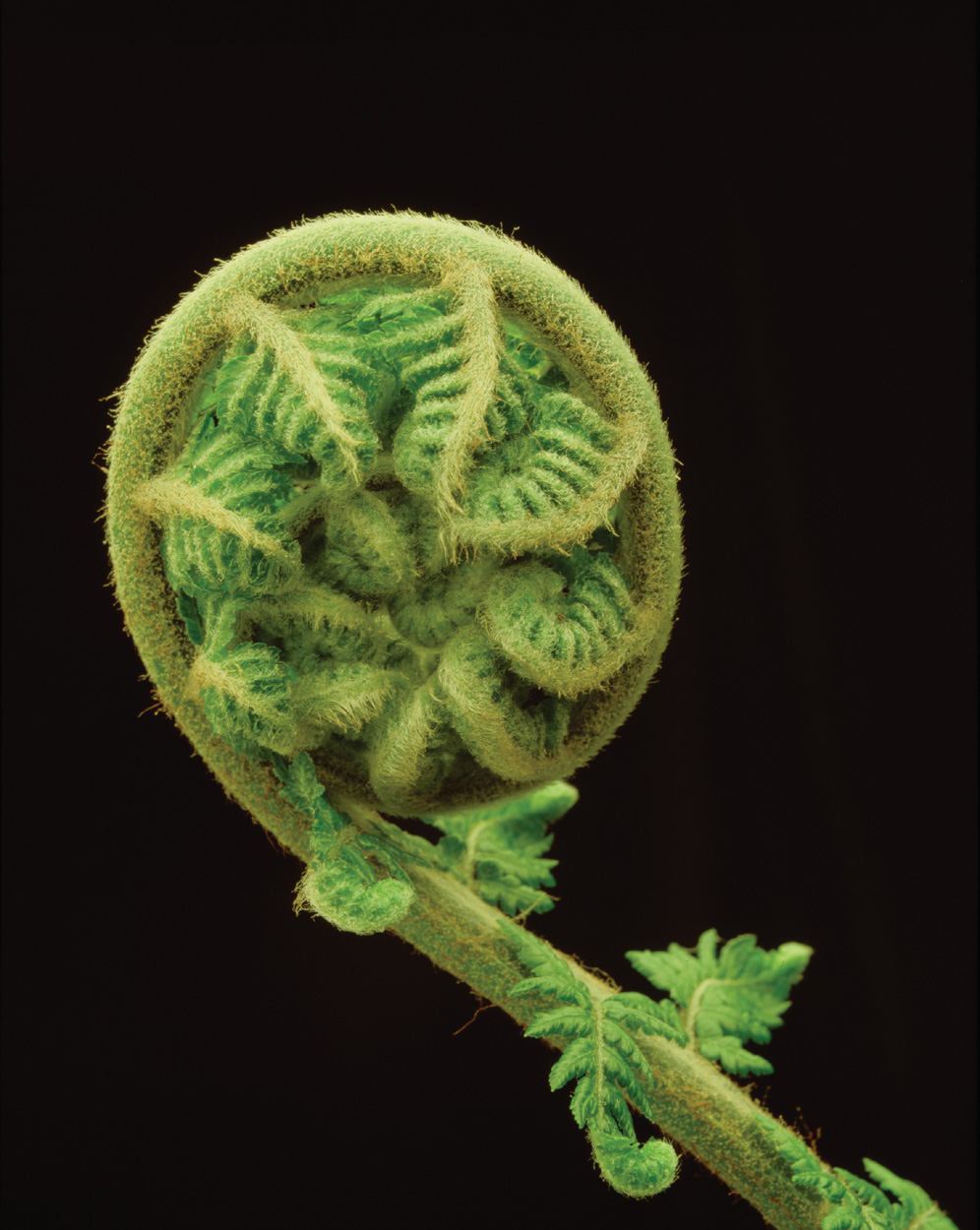
Ferns and horsetails form a monophyletic group that are the sister group to the seed plants (Fig. 33.11). The majority of species in this group are in fact ferns, which can be recognized by their distinctive leaves (Fig. 33.11) that uncoil during development from tightly wound “fiddleheads” (Fig. 33.12). This group also includes horsetails and whisk ferns, which traditionally were considered as distinct groups because of their unique body organizations. Whisk ferns have photosynthetic stems without leaves (see Fig. 33.11b) and they also do not form roots. They thus look similar to fossils of early vascular plants. Molecular sequence comparisons, however, support the hypothesis that whisk ferns are the simplified descendants of plants that produced both leaves and roots and that they are members of the same lineage as ferns.
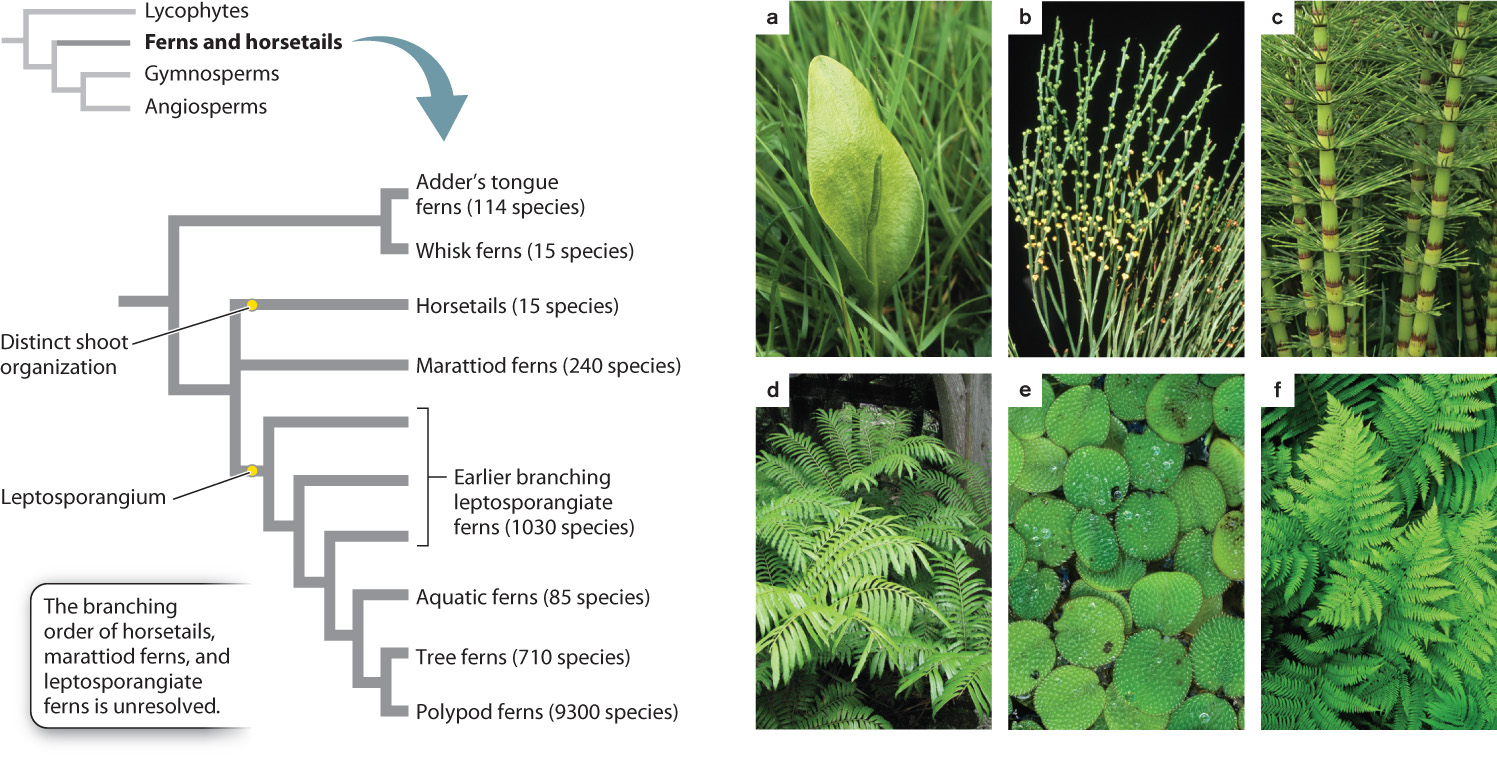
The horsetails, represented by 15 living species of the genus Equisetum, produce tiny leaves arranged in whorls, giving the stem a jointed appearance (see Fig. 33.11c). Horsetail stems are hollow, and their cells accumulate high levels of silica, earning them the name “scouring rush.” Today, horsetails are small plants, most less than a meter tall. However, tree-sized versions grew side by side with the ancient lycophyte trees (see Fig. 33.9).
Fern leaves can be large—in some cases up to 5 m long, although typically the photosynthetic surfaces are divided into smaller units called pinnae. Fern stems frequently grow underground, and only the leaves emerge into the air. Internode elongation can be very short, so that all the leaves are bunched together. Some species, such as bracken ferns, produce horizontal stems with widely spaced leaves. This arrangement allows bracken to spread rapidly. Because bracken is toxic to cattle and other livestock, its tendency to invade pastures is a serious problem.
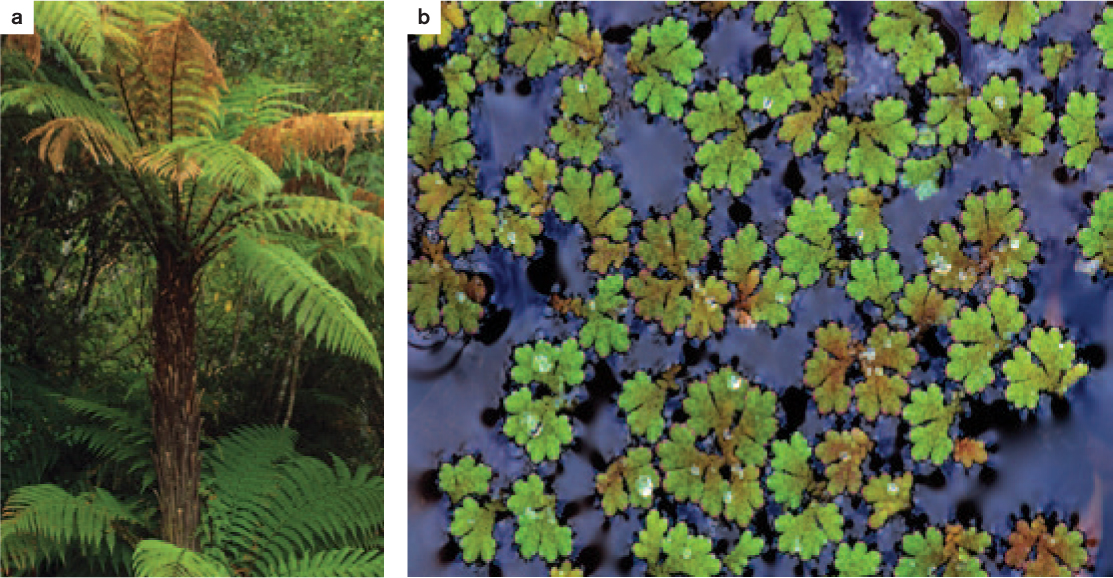
Vascular tissue allows fern sporophytes to grow large. However, because ferns do not exhibit secondary growth, there are limits to how tall they can grow. Tree ferns can grow to more than 10 m by producing thick roots that descend from the leaves to the ground, adding both vascular capacity and mechanical support (Fig. 31.13a). Other ferns grow tall by producing leaves that twine around the stems of other plants, using the other plant for support. At the other extreme are tiny aquatic ferns such as Salvinia (see Fig. 33.11e) and Azolla (Fig. 33.13b) that float on the surface of the water.
Horsetails, whisk ferns, and a few other fern groups make large sporangia similar to those produced by early vascular plants. Most ferns, however, have a distinctive sporangium (called a leptosporangium) whose wall is only a single cell thick. Polypod ferns (see Fig. 33.11f), in particular, have leptosporangia with a line of thick-walled cells that run along the sporangium surface. When the spores mature, the sporangium dries out and these cells contract, forcibly ejecting spores into the air. Polypod ferns are by far the most diverse group of ferns (about 9300 species), and their distinctive sporangia, with their high potential for dispersal, may have played a role in their evolutionary success.
Although the fossil record shows that ferns originated more than 360 million years ago, the radiation of the polypod ferns occurred after the rise of the angiosperms. Thus, many of the fern species present today are likely to have evolved to occupy habitats newly created by the development of angiosperm forests. Approximately 40% of living ferns are epiphytes, many of them growing in tropical rain forests dominated by angiosperm trees.
Epiphytic ferns are exposed to much more light than plants established on the ground. However, the canopy environment poses its own challenges, notably the danger of dislodgement and the risk of drying out. As a result, epiphytic ferns have adaptations that allow them to survive in the forest canopy. For example, staghorn ferns (Fig. 33.14) produce two leaf types: erect leaves that are photosynthetic and produce sporangia and basal leaves that grow close to the stem of the host tree, forming a pocket in which organic matter accumulates. The roots of staghorns can grow into the pocket of organic matter, where they are protected from damage and desiccation.

33.3.5 An aquatic fern contributes to rice production.
Azolla is a free-floating aquatic fern that has been used for centuries in Asia as a biofertilizer of rice paddies (see Fig. 33.13b). Its success as a fertilizer is due to its symbiotic association with nitrogen-fixing cyanobacteria. Azolla is common worldwide on still water such as ponds and quiet streams. Its leaves contain an internal cavity in which colonies of nitrogen-fixing cyanobacteria become established. The cyanobacteria provide Azolla with a ready source of nitrogen, while the fern provides shelter and carbohydrates. Rates of nitrogen fixation by the cyanobacteria are reported to be greater when the cyanobacteria are housed within an Azolla leaf than when these organisms live freely in the environment.
When added to newly planted rice fields, this fast-growing fern suppresses weed growth by forming a dense, floating layer. At the same time, it provides organic nitrogen that eventually finds its way into the developing rice plants. The floating fertilizer factories provided by Azolla may have made possible the long history of rice cultivation in Asia.