34.1 GROWTH AND NUTRITION
Fungi are heterotrophs, meaning that they depend on pre-existing organic molecules for both carbon and energy. Unlike animals, fungi do not have organs that enable them to ingest food and break it down in a digestive cavity (Chapter 40). Instead, fungi absorb organic molecules directly through their cell walls. This mode of feeding presents two major problems. First, although simple molecules like amino acids and sugars pass readily across the cell wall, more complicated molecules do not. Fungi secrete a diversity of enzymes that break down complex organic molecules like starch, cellulose, or lignin into simpler compounds that can be absorbed. Fungi, then, digest their food first and take it into the body afterward.
A second challenge is to find food in the environment. Other heterotrophic organisms such as bacteria, protists, and animals commonly move through their habitat, actively searching for food. Fungi, however, have no means of locomotion, and so these organisms use the process of growth itself to find nourishment.
34.1.1 Hyphae permit fungi to explore their environment for food resources.
Most fungi are multicellular, consisting of highly branched filaments called hyphae (Fig. 34.1). The hyphae are slender, typically 10 to 50 times thinner than a human hair. The numerous long, thin hyphae provide fungi with a large surface area for absorbing nutrients.
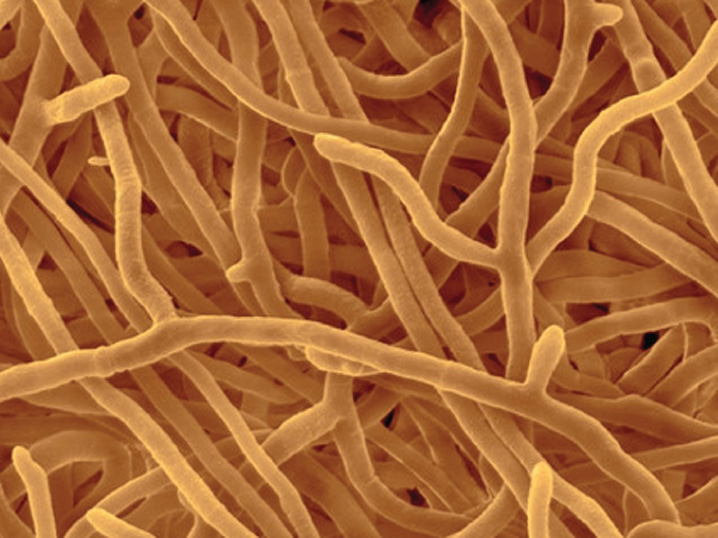
Hyphae maintain their slender form by growing only at their tips. Elongating hyphae thus penetrate ever farther into their environment, encountering new food resources as they grow. Where resources are low, growth is slow or may stop entirely. On the other hand, when fungi encounter a rich food resource, they grow rapidly and branch repeatedly, forming a network of branching hyphae called a mycelium. Mycelia can grow to be quite large—the largest known individual of the fungus Armillaria ostoyae covers over 2000 acres in the Blue Mountains of Oregon and weighs many hundreds of tons. We may be impressed by blue whales and redwoods, but in fact some fungi are the largest living organisms on Earth.
A strong but flexible cell wall is key to hyphal growth and nutrient transport. In fungi, cell walls are made of chitin, the same compound as found in the exoskeletons of insects. Chitin is a modified polysaccharide that contains nitrogen. Fungal cell walls are thinner than plant cell walls because of the chemical makeup of chitin. The wall prevents cells from rupturing when exposed to dilute solutions such as those in freshwater environments and in the soil. Moreover, the walls keep cells from expanding as water flows into the cytoplasm by osmosis. This inflow of water generates positive turgor pressure (Chapters 5 and 29), providing the force that enables fungi to explore their surroundings as the hyphal tip is pushed ever deeper into the local environment.
34.1.2 Fungi transport materials within their hyphae.
Fungi can transport food and signaling molecules across long distances in mycelia, so they are able to grow between resource patches and to produce reproductive structures such as mushrooms that rise up above the ground. Molecules taken up actively from the environment drive water into the cell by osmosis and thus increase turgor pressure. At the same time, growth and respiration consume these molecules, resulting in a decrease in turgor pressure. Such differences in turgor pressure along hyphae drives bulk flow in a manner similar to phloem transport in plants (Chapter 29). Bulk flow carries materials obtained in a nutrient-rich location so they can fuel hyphal elongation across nutrient-poor locations. Transport by bulk flow also allows fungi to build relatively large reproductive structures aboveground. All the raw materials used to build a mushroom must be transported from hyphae in contact with the soil or a rotting log.
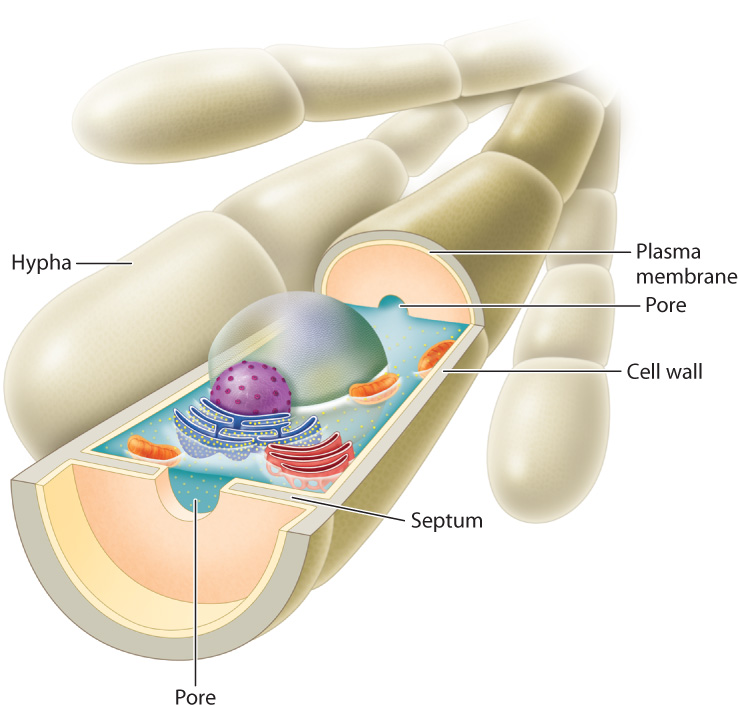
Cytoplasmic continuity is essential for the long-distance movement of materials within mycelia. In early-diverging groups, the hyphae have many nuclei but no cell walls to separate them. In later-diverging groups, nuclear divisions are accompanied by the formation of septa, walls that partially divide the cytoplasm into separate cells (Fig. 34.2). Each septum contains one or more pores that allow water and solutes to move freely between cells. Septa play an important role when hyphae are damaged. Injury activates sealing mechanisms that plug pores in the septa, preventing the loss of pressurized cytoplasm.
Question Quick Check 1
SfgnTmyjMlyZPREzeuP5e3DnRNOXdW4frXeIBcH+DtY=34.1.3 Not all fungi produce hyphae.
Yeasts are single-celled fungi found in moist, nutrient-rich environments. In these circumstances, hyphae, which are used to search for food, are unnecessary. All yeasts are descended from ancestors that developed hyphae, but hyphal development has been lost independently several times. Thus, the lack of hyphae is an example of convergent evolution, in which the same trait develops independently in different lineages in response to similar selective pressures.
Most yeasts divide by budding (Chapter 42). A small outgrowth increases in size and eventually breaks off to form a new cell (Fig. 34.3). The localized outgrowth that results in budding is similar to growth by elongation at hyphal tips. In fact, some yeasts can form hypha-like structures under certain conditions. For example, in Candida albicans, a yeast that infects humans, hyphae are produced during the transition of the fungus from a harmless to a pathogenic form.
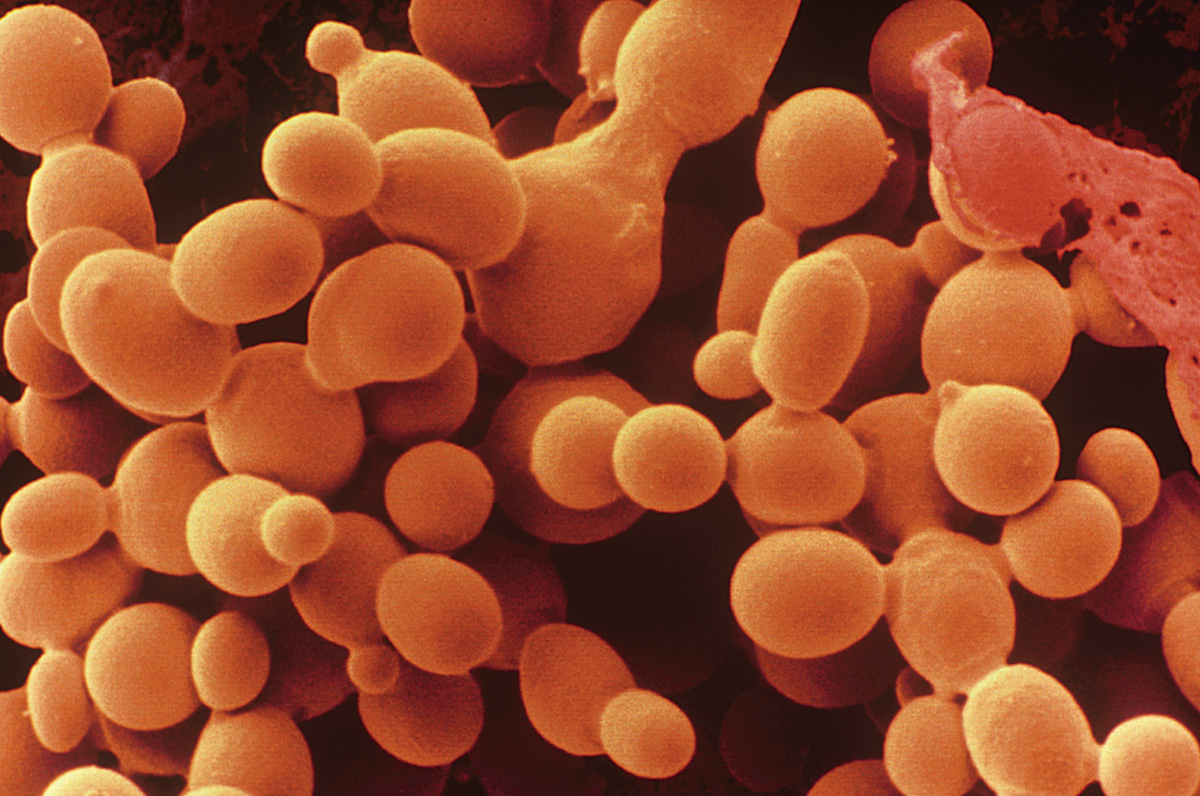
Yeasts are common on the surfaces of plants, and to a lesser extent on the surfaces and in the guts of animals. Humans have long used the yeast Saccharomyces cerevisiae to ferment plant carbohydrates to produce leavened bread and alcoholic beverages. S. cerevisiae is also an important model organism used in laboratory studies of genetics.
34.1.4 Fungi are principal decomposers of plant tissues.
Most fungi use dead organic matter as their source of energy and raw materials. As noted in Chapter 25, on land the organic matter in dead tissues on and within soils far exceeds the amount in the living biomass. Thus, the ground beneath our feet provides tremendous resources for heterotrophic growth. Bacteria and some protists can use this resource, but for the most part it is the fungi that convert dead organic matter back to carbon dioxide and water. This helps to keep the biological carbon cycle in balance (Chapter 25) and returns nutrients in leaves to the soil where they will be available for new plant growth.

A brief consideration shows why fungi have the advantage on a forest floor or in soil. Like fungi, bacteria secrete enzymes that break down organic molecules in their immediate environment, but in the soil bacteria have only a limited capacity to move from place to place. Flagella can propel them a few micrometers through a thin film of water, but when local resources become depleted, bacteria must wait for new food to appear. In contrast, hyphal growth allows fungi to search actively through the soil for new food resources. Furthermore, because fungi can penetrate their food, they are particularly well suited for breaking down the bodies of multicellular organisms, such as the stems of trees.
Fungi can gain nutrition from dead animal, protozoan, or even bacterial cells, but the most abundant biomolecules on and within soils are cellulose and lignin, the principal components of plant cell walls (Chapter 31). Cellulose in particular is a rich source of carbon and energy. Cellulose is difficult to degrade because individual cellulose polymers bind tightly to one another. In addition, in wood and other lignified plant tissues, cellulose microfibrils occur in intimate association with lignin, making the cellulose even harder to get at. Lignin is even more difficult for enzymes to break apart, in part because it lacks a regular chemical structure. A few bacteria are known to decompose lignin, but in nature, fungi account for most of the decomposition of wood (Fig. 34.4).
To break down lignified plant cell walls, fungi require oxygen. However, oxygen concentrations are typically low in waterlogged environments, and in that case decay proceeds slowly if at all. For this reason, woody biomass, once submerged in water, tends to accumulate, leading to the buildup of partially decayed plant material as peat. Over geologically long time intervals, peat becomes coal, an energy staple of the industrial world that owes its existence to the inhibition of fungal decay.
34.1.5 Fungi are important plant and animal pathogens.
Fungi not only feed on dead plants and animals, but they are also well adapted to infect living tissues (Fig. 34.5). Plants, for example, are vulnerable to a diverse array of fungal pathogens— rusts, smuts, and molds—that cause huge losses in agricultural production, as we discuss in section 34.4.
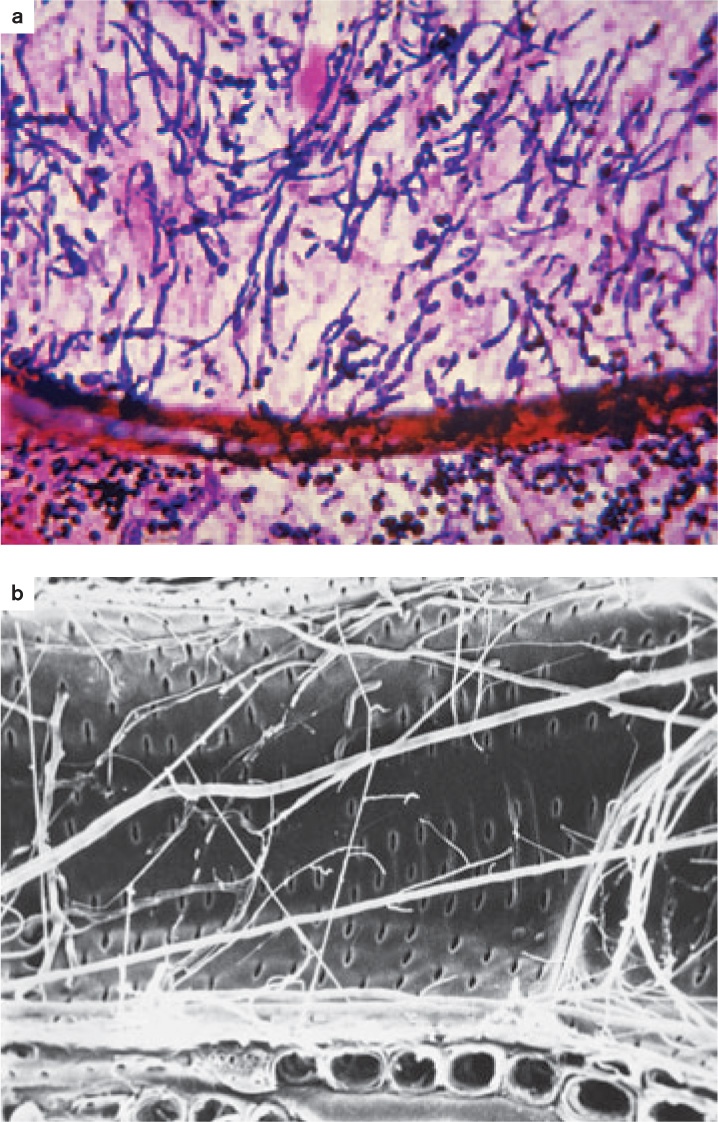
Invasion is the key to success for plant pathogens (Chapter 32). Most plants have physical or chemical means of inhibiting fungal invasion, so successful pathogens must be able to get past these defenses. In many cases, fungi infect plants through wounds, which provide a route around a plant’s outer defenses. Some fungi enter through stomata, while others penetrate epidermal cells directly, degrading the wall with enzymes and then pushing their hyphae into the plant interior by turgor pressure. The extent of infection usually depends on the ability of hyphae to expand into new tissues. Vascular wilt fungi (discussed in Chapter 32) send their hyphae through xylem vessels, enabling them to spread throughout the entire plant. Old photographs of towns in the eastern United States show streets shaded by American elm trees, but today nearly all those trees are dead, victims of a vascular wilt fungus introduced into the country early in the twentieth century.
Aboveground, plant infections are usually transmitted by fungal spores, carried either by the wind or on the bodies of insects. For example, many fungi that attack fruits are transmitted by insects also seeking those fruits; yeasts that feed on nectar within flowers hitch rides on pollinators. Some fungi secrete sugar-rich droplets that attract insects, which then transport their spores to other sites.
Belowground, infection is typically transmitted by hyphae that penetrate the root. Hyphae can feed on organic matter in the soil until the root of a suitable host plant is encountered. The persistence of fungal hyphae in soils means that populations of pathogenic fungi can build up in the soil. This is why seedlings germinating close to an adult tree of the same species are at risk of infection, whereas seedlings of distantly related species may be spared. By favoring the establishment of seedlings of less-common species, fungal pathogens are thought to play an important role in maintaining high levels of species diversity in tropical rain forests (Chapter 32).
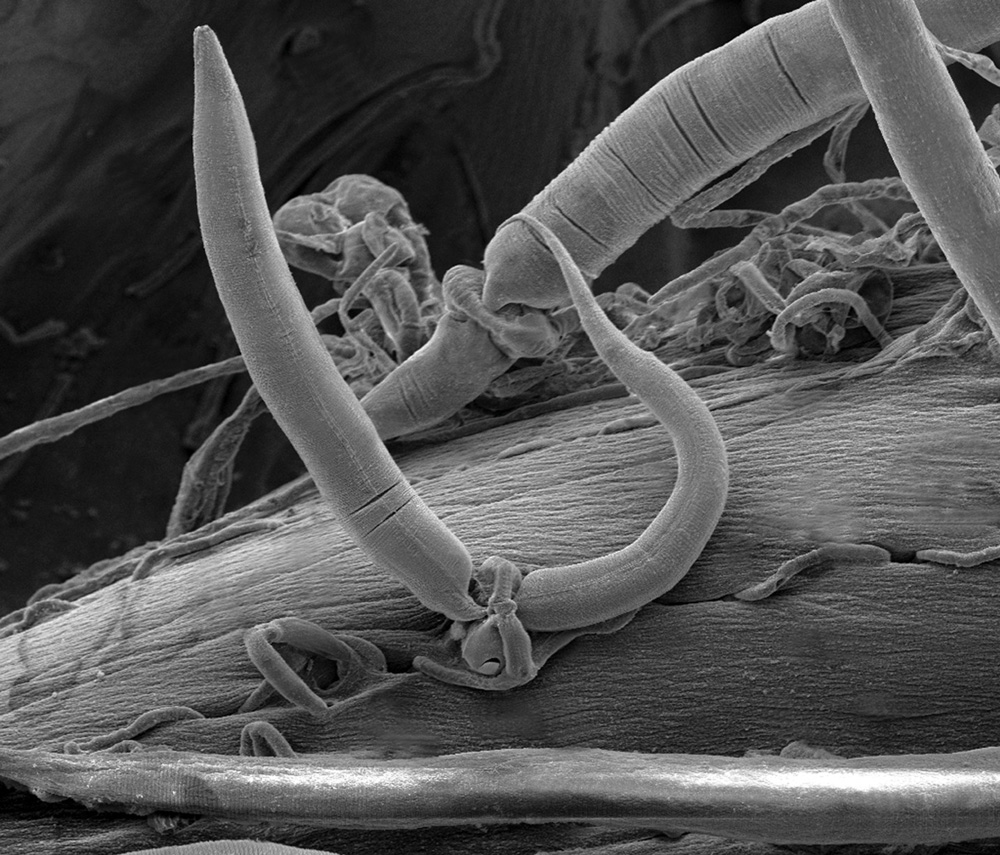
Insects and other invertebrates are also susceptible to fungal pathogens. Certain fungi exploit living animals in a unique way: by trapping them. Some fungal predators form sticky traps with their hyphae, whereas others actually lasso their prey (Fig. 34.6). The hyphae of these fungi form rings that can inflate within seconds, trapping anything within the ring. Nematodes, tiny worms common in soils, inadvertently pass through the rings, setting off hyphal inflation. The nematodes are then trapped, providing food for this fungus.
Fungal infection is relatively rare in vertebrates. Severe infections are more frequent among fish and amphibians than in mammals, perhaps because fungi grow poorly at mammalian body temperatures. An apparent exception is the fungal infection that has caused dramatic declines in North American bat populations, but the fungi infect the bats during their winter hibernation, when the bats have lowered body temperatures that conserve energy. In humans, most fungal infections are annoying rather than life threatening—athlete’s foot and yeast infections are prime examples. However, in individuals with immune systems compromised by HIV or other illnesses, fungal infection can be fatal.
34.1.6 Many fungi form symbiotic associations with plants and animals.

While some interactions between species benefit the fungus at the expense of its host, others benefit both partners. In Chapter 29, we saw that mycorrhizal fungi supply plant roots with nutrients such as phosphorus from the soil and in return receive carbohydrates from their host (Fig. 34.7). There are two main types of mycorrhizae. The hyphae of ectomycorrhizal fungi surround, but do not penetrate, root cells. In contrast, the hyphae of endomycorrhizal fungi penetrate into root cells, where they produce highly branched structures that provide a large surface area for nutrient exchange. As we will see in section 34.3, the endomycorrhizal fungi constitute a monophyletic group dating back over 400 million years. In contrast, ectomycorrhizae are present in multiple groups and appear to have evolved more than once.
Other fungi, called endophytes, live within leaves. While endophytes are much less studied than mycorrhizae, they may be as widespread. Endophytic hyphae grow within cell walls and in the spaces between cells. Long thought to be harmless, endophytes are now recognized as beneficial. They may help the host plant by producing chemicals that deter pathogens and herbivorous insects.
Mutually beneficial associations between fungi and animals are much less common, but a few examples are known, none more striking than insects that actually grow fungi for food. The fungi benefit because the insects provide shelter, food, and protection from predators and pathogens. Insect-fungal agriculture has evolved at least three times: in leaf-cutter ants that live in tropical forests (Chapter 48), in a group of African termites, and in some wood-boring beetles. These beetles maintain fungal gardens within tunnels that they excavate in damaged or dead trees.
34.1.7 Lichens are symbioses between a fungus and a green alga or a cyanobacterium.
Lichens are familiar sights in many environments, often forming colorful growths on rocks or tree trunks (Fig. 34.8). Lichens look, function, and even reproduce as single organisms, but they are actually stable associations between a fungus and a photosynthetic microorganism, usually a green alga but sometimes a cyanobacterium. Nearly 15% of all known fungal species grow as lichens.

The dual nature of lichens was first proposed by a Swiss botanist, Simon Schwendener, in 1867, but his hypothesis found little favor at the time. The existence of a composite “organism” challenged the idea that life could be divided into discrete categories such as animals and plants and raised the question of how an association of distinct species could function as an integrated whole. Given what we now understand about the widespread nature of mutualisms, the opposition to Schwendener’s proposal may seem surprising. Yet it was only in 1939, when Eugen Thomas showed that lichens could be separated into their individual parts and then reassembled, that their dual nature became widely accepted.
Lichens consist mostly of fungal hyphae, with the photosynthetic algae or cyanobacteria forming a thin layer just under the surface (Fig. 34.9). The hyphae anchor the lichen to a rock or tree, aid in the uptake and retention of water and nutrients, and produce chemicals that protect against excess light and herbivorous animals. In turn, the photosynthetic partners provide a source of reduced carbon, such as carbohydrates. Cyanobacteria living in lichens also provide a source of fixed nitrogen, like ammonia. The two partners exchange nutrients through fungal hyphae that tightly encircle or even penetrate the walls of the photosynthetic cells. As a result, lichens are able to thrive at sites where neither partner could exist on its own.
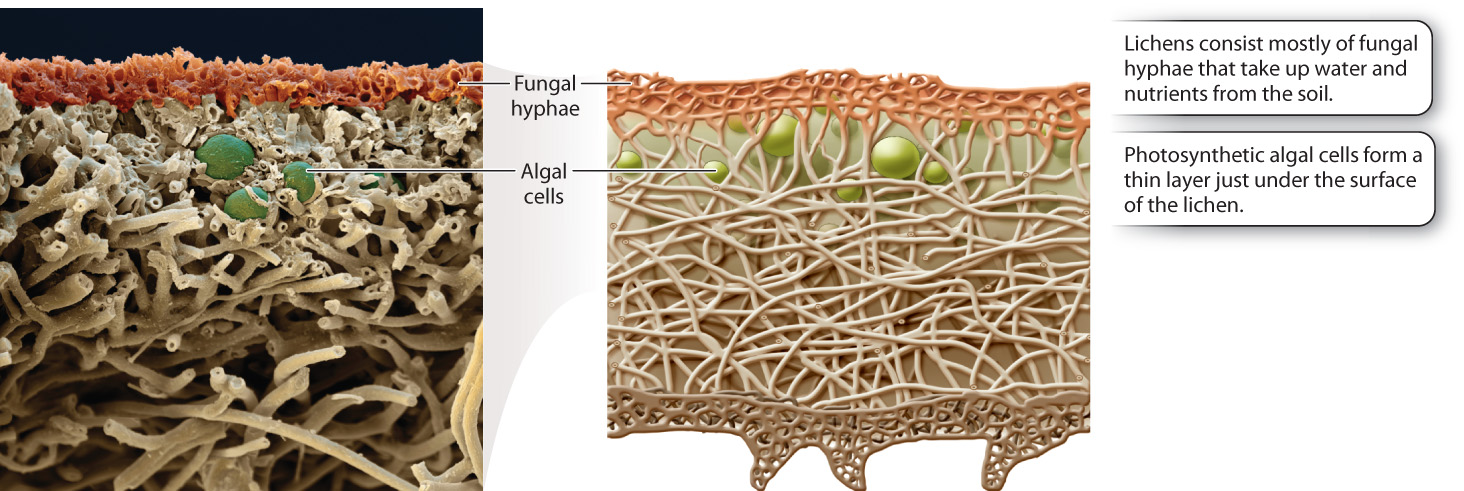
It remains an open question whether either partner can exist independently in nature. In cases where the fungal partner can be grown in the laboratory in culture, it produces a relatively undifferentiated hyphal mass. Thus, while the bulk of the lichen structure comes from the fungus, chemical signals from the photosynthetic partner influence the form and shape of the fungus.
Surprisingly, these associations are not species specific. There are approximately 13,500 known lichens, but only about 100 participating photosynthetic species. This means that different lichens can have the same algae or cyanobacteria. Conversely, a given fungal partner may associate with more than one photosynthetic species, without affecting the lichen’s outward form. Because of the intimate association between lichen morphology and fungal species, the lichen and fungus are assigned the same scientific name.
Lichens spread asexually by fragmentation or through the formation of dispersal units consisting of a single photosynthetic cell surrounded by hyphae. Sexual reproduction, at least by the fungi, is common, and the photosynthetic cells reproduce asexually by mitotic cell division. Whether sexual or asexual reproduction is more important in allowing lichens to establish in new habitats, however, is not known.
Lichens are remarkable for their ability to grow on the surfaces of rocks and tree trunks. They are among the first colonizers of lava flows and the barren land left after glacial retreat. In some habitats, for example Arctic tundra and semiarid landscapes, lichens make a measurable contribution to net productivity. How do lichens obtain the resources they need in these barren environments? They obtain nutrients from rainfall or by secreting organic acids that help release some nutrients from even rocky surfaces. Not surprisingly, lichens in these harsh environments grow slowly. Some lichens grow on soil, for example reindeer “moss,” which is eaten by caribou. Reindeer moss is thought to compete successfully for space by secreting chemicals that hinder the growth of plants.
Given their small size and exposure to the environment, lichens must be able to tolerate drying out from time to time. All lichens have a high tolerance for dessication, and can tolerate wide fluctuations in temperature and light. In contrast, lichens are quite sensitive to air pollution, particularly sulfur dioxide (SO2). For this reason, lichen growth is sometimes used as an indicator of industrial pollution.