34.2 REPRODUCTION
Like plants, fungi face two challenges in completing their life cycles. First, to maintain genetic diversity within populations, they must find other individuals with which to mate. Second, they must be able to disperse from one place to another. The ways that fungi generate genetic diversity are unique. However, fungal adaptations for dispersal at least broadly resemble those of plants. Fungi rely on wind, water, or animals to carry spores through the environment. Recall from Chapter 30 that spores are specialized cells well adapted for dispersal and long-term survival.
We most often come into contact with fungi through their reproductive structures. Fungal spores commonly cause respiratory illness, and mushrooms attract our attention because they are delicious or poisonous. Yet many aspects of fungal reproduction are not easily observed, including life-cycle features that distinguish fungi from other eukaryotes. Fungi have a diverse set of reproductive strategies. In this section, we emphasize the most general principles and patterns.
34.2.1 Fungi proliferate and disperse using spores.
The tissues, living or dead, that support fungal growth are sometimes hard to come by and have a patchy distribution. For this reason, fungi must be able to disperse from one food source to another. The extensive networks of hyphae within soils or host organisms can spread locally but cannot disperse over great distances, so fungi produce spores that can be carried by the wind (Fig. 34.10a), in water, or attached to (or within) animals. In early-diverging fungi that still live in aquatic environments, spores have flagella that allow them to swim. The great majority of fungi, however, live on land, and their spores have no flagella. Instead, the spores are encased in a thick wall that protects them as they are dispersed over habitats unsuitable for growth.
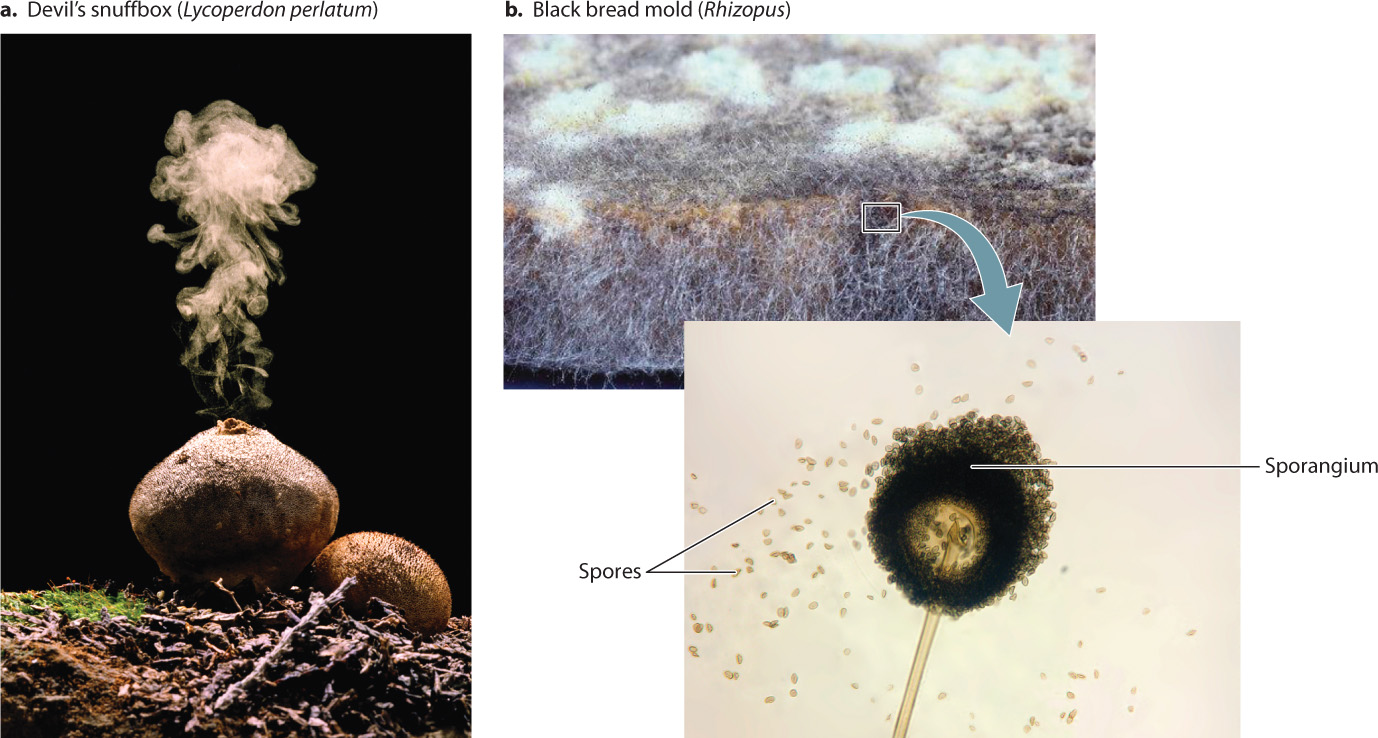
The probability that any given spore will come to rest in a favorable habitat is low, so fungi produce huge numbers of spores. Fungal spores remain viable, able to grow if provided with an appropriate environment, for periods ranging from only a few hours in some species to many years in others. Thus, spores allow fungi to use resources that are patchy in time as well as in space. In fact, a shortage of resources is one of the cues that triggers spore formation.
Spores can form by meiotic cell division as part of sexual reproduction, and they can also form asexually. Asexual spores are formed by mitotic cell division and therefore are genetically identical to their parent. Asexual spores allow fungi to proliferate and disperse to new environments. In many species, asexual spores are produced within sporangia that form at the ends of erect hyphae, facilitating the release of the spores into the air. A close look at a moldy piece of bread reveals that the surface is covered with hyphae carrying sporangia containing asexual spores (Fig. 34.10b).
Fungal species vary in the extent to which they rely on asexual as opposed to sexual spore production. In some groups, such as the lineage that includes the most common mushrooms, sexual reproduction is the dominant means of spore production. On the other hand, in a small number of fungi that includes the endomycorrhizal species, spores produced by meiotic cell division have never been observed. Although sexual reproduction appears to be absent in some fungal species, asexual fungi have other mechanisms for producing genetic diversity, as discussed later in this section.
34.2.2 Multicellular fruiting bodies facilitate the dispersal of sexually produced spores.
Fungi employ an astonishing array of mechanisms to enhance spore dispersal. Particularly conspicuous are the multicellular fruiting bodies produced by some fungi, which facilitate the dispersal of sexually produced spores. (Fungal fruiting bodies should not be confused with the fruits of flowering plants, which are unrelated and very different structures.) Mushrooms, stinkhorns, puffballs, bracket fungi, truffles, and many other well-known structures are fungal fruiting bodies.

Fruiting bodies are highly ordered and compact structures compared to the mycelia from which they grow, yet they are constructed entirely of hyphae (Fig. 34.11). In fact, in many cases their mechanisms of spore dispersal demand a high degree of structural precision. Yet we know little of the developmental processes that allow tip-growing hyphae to produce such complex and regular structures.
The fruiting bodies of many fungi rise above the ground or grow from the trunks of dead trees, so the sexually produced spores are released high above the ground. But elevation by itself is not enough to ensure dispersal. Thus, many fungi forcibly eject their spores, achieving velocities of more than 1 m/s that allow the tiny spores to penetrate and travel beyond the layer of stagnant air that surrounds the fruiting body. Studies have shown that spores move efficiently through air (Fig. 34.12). Other fungi rely on external agents such as raindrops or animals to move their spores around. In section 34.3 we will encounter examples of the diverse dispersal mechanisms found in the fungi.
FIG. 34.12: What determines the shape of fungal spores that are ejected into the air?
BACKGROUND Many fungi disperse their spores by ejecting them forcibly into the air. For spores to be picked up by the wind, however, they must escape a layer of still air called the boundary layer that lies close to the ground. Escape from the boundary layer is easier if spores have a size and shape that minimize drag, the resistance of air to the movement of an object.
HYPOTHESIS In fungi that eject their spores into the atmosphere, natural selection favors spore shapes that minimize drag.
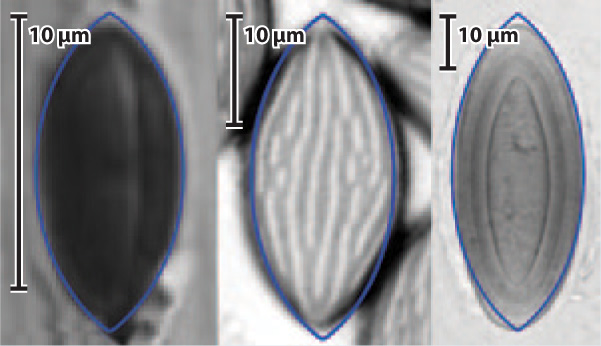
EXPERIMENT working from computer models for minimizing drag on airplane wings, scientists modeled spore shapes that minimize drag. these models took into account spore size as well as the physical characteristics of the air through which spores travel. the scientists then measured spore shape for more than 1 00 species of spore-ejecting fungi.
RESULTS Nearly three-quarters of the examined fungi had spore shapes that came within 1% of the shape calculated to minimize drag. Related species that do not eject spores forcibly were less likely to have drag-minimizing shapes.
CONCLUSION Natural selection has acted on fungi to facilitate spore dispersal by wind.
SOURCE Roper, M., R. E. Pepper, M. P. Brenner, and A. Pringle. 2008. “Explosively Launched Spores of Ascomycete Fungi Have Drag-Minimizing Shapes.” Proceedings of the National Academy of Sciences, USA 105:20583-20588.
34.2.3 The fungal life cycle often includes a stage in which haploid cells fuse, but nuclei do not.
Like other sexually reproducing eukaryotes, fungi have life cycles that include haploid (1n) and diploid (2n) stages. The nuclei in fungal hyphae are haploid, and the fungal life cycle is therefore similar to haploid-dominant life cycles found among eukaryotic organisms (see Fig. 27.3a). In haploid-dominant organisms, asexual reproduction involves the production of haploid spores by mitosis, while sexual reproduction involves the fusion of haploid cells (often differentiated as gametes) to form a diploid zygote, which undergoes meiosis as its first division. However, sexual reproduction in fungi differs from all other haploid-dominant organisms in one important respect: In fungi, the fusion of haploid cells is not immediately followed by the fusion of their nuclei.
In most fungi, the sexual phase of the life cycle involves the fusion of hyphal tips rather than specialized reproductive cells, or gametes. For mating to occur, two hyphae grow together and release enzymes that digest their cell walls at the point of contact. This allows the cell contents of the two hyphal cells to merge, forming a single cell with two haploid nuclei. In most sexually reproducing organisms, when two gametes merge, their nuclei fuse almost instantly to form a diploid zygote. In fungi, however, the cytoplasmic union of two cells (plasmogamy) is not always followed immediately by the fusion of their nuclei (karyogamy). Instead, the haploid nuclei retain their independent identities, resulting in what is referred to as a heterokaryotic (“different nuclei”) stage (Fig. 34.13). In the heterokaryotic stage, cells have nuclei from two parental hyphae, but the nuclei remain distinct. The heterokaryotic stage ends with karyogamy, which leads to the formation of a diploid zygote. The zygote divides by meiotic cell division, giving rise to sexually produced haploid spores.
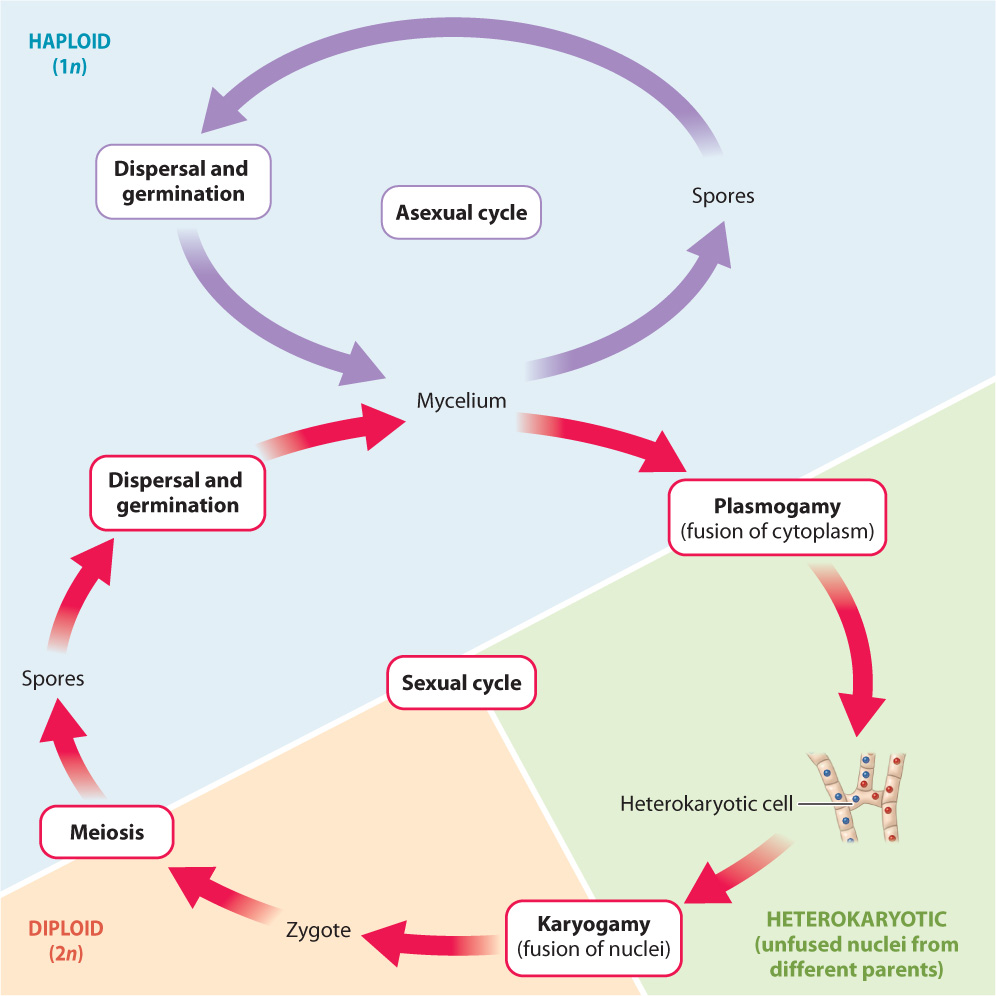
The separation between plasmogamy and karyogamy is unique to the fungi, and as will be discussed in the next section, the elaboration of this difference is a major theme in the evolution of fungi. In the earliest diverging fungal lineage, karyogamy follows fast on the heels of plasmogamy and there is no heterokaryotic stage. In some groups, the heterokaryotic stage consists of a single cell with many haploid nuclei. However, in other groups, plasmogamy is followed by mitosis, which produces hyphae in which each cell contains two haploid nuclei, one from each parent. Heterokaryotic cells with just two genetically distinct haploid nuclei are referred to as dikaryotic, or n + n, cells. In fungi with dikaryotic cells, called dikaryotic fungi, there may be a small number of dikaryotic cells or extensive hyphal development made up of dikaryotic cells. For example, some edible mushrooms found on market shelves consist entirely of dikaryotic hyphae.
Dikaryotic fungi account for more than 98% of all known fungal species, suggesting that the separation of plasmogamy and karyogamy in time and space may confer an evolutionary advantage. One major advantage can be seen when we think about the environments where fungi live. Mating takes place principally within the soil or inside the trunks of rotting trees, sites where hyphae are most likely to come into contact. Dispersal, however, is most effective when spores can be released into the air. The separation of plasmogamy and karyogamy allows mating and spore production to occur where each is most effective. In some dikaryotic fungi, however, the separation of plasmogamy and karyogamy is neither distant in space nor long in time. In these fungi, the highly branched dikaryotic hyphae may serve primarily as a means of increasing the number of cells in which karyogamy will eventually take place, therefore leading to the production of more sexually produced spores.
At present, we really don’t know why fungi proliferate n + n cells rather than forming a multicellular diploid phase, and there may be no single answer to the question of why the dikaryotic fungi have this unique cell type.
Question Quick Check 2
sPEJ8G2ATIcCmHZK3ElSLXYnb+sqXUTfvTr0QLV6YeVEd6rCPW3EhmsdKmk4UFduY6MPTXZAeGDA897Ylt+DAyrjZt4QMdLt34.2.4 Genetically distinct mating types promote outcrossing.
Under most conditions found in nature, genetically diverse populations persist better than those lacking diversity. Indeed, sexual reproduction is widely viewed as a means of promoting genetic diversity for long-term ecological success in variable environments (Chapters 11 and 42). With the exception of early-diverging aquatic lineages, fungi do not produce male and female gametes. How, then, is sex accomplished, and what prevents an individual from mating with itself?
Many individual fungi of a species look similar but nevertheless have different mating types that are genetically determined and prevent self-fertilization. The mating type of an individual is determined by a mating-type gene. Fertilization can take place only between individuals that have different alleles at the mating type gene. In some species, there are only two mating-type alleles. In this case, mating patterns are identical to those for species with male and female sexes. If the two mating-type alleles are spread evenly throughout the population, an individual should be able to mate with 50% of the general population.
Some fungi have more than two mating-type alleles. These fungi have a greater likelihood of encountering a compatible genotype in the general population. That likelihood is even higher for fungi in which mating type is determined by two different mating-type genes that each have multiple alleles. Fungi in the group that includes the common mushrooms can have as many as 20,000 different mating-type alleles. In that case, the odds of finding a compatible mate are close to 100%.
Genetic compatibility is a prerequisite for mating. How do fungi go about finding a suitable mate? The answer is that they secrete chemical signals that attract fungi with mating types different from their own. The chemical nature of these signals differs among groups.
34.2.5 Fungi that lack sexual reproduction have other means of generating genetic diversity.
Asexual reproduction occurs in most groups of fungi. Indeed, about 20% of fungi appear to lack sexual reproduction altogether. Among these are such well-known groups as Pénicillium (the source of the antibiotic penicillin), Aspergillus (the major industrial source of vitamin C), and all the endomycorrhizal fungi.
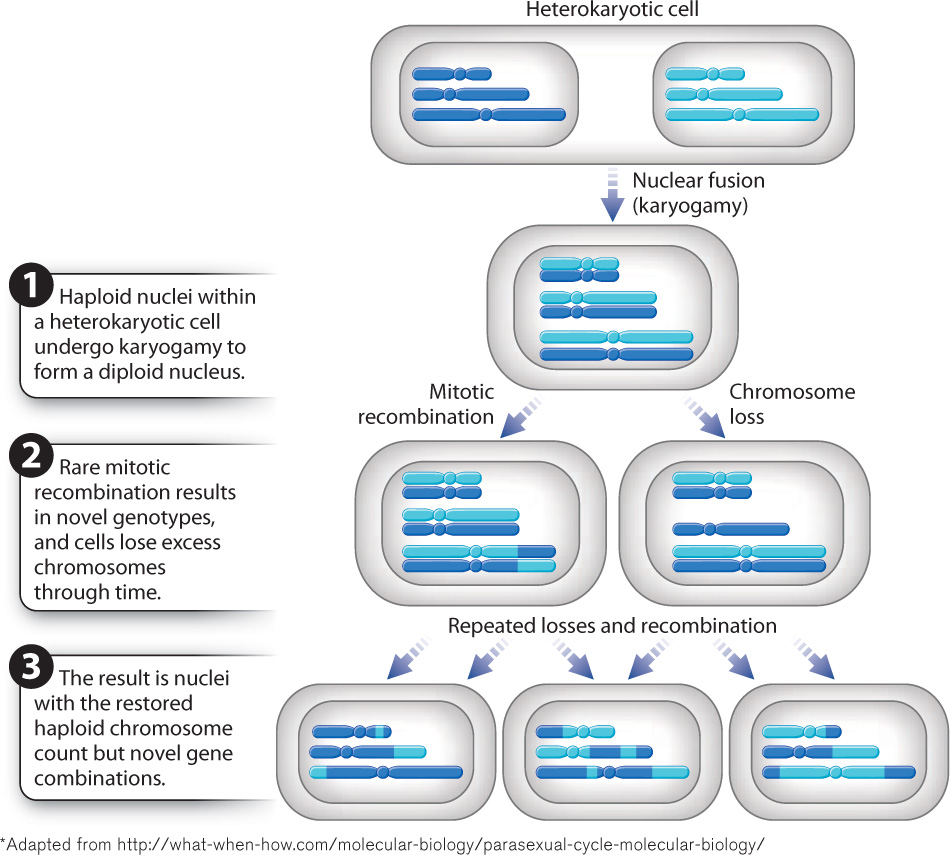
How are asexual species able to persist without a mechanism for generating genetic diversity? The short answer is, they don’t. Fungal species that lack an observable sexual cycle have another mechanism of generating genetic diversity: the crossing over of DNA during mitosis. Such species are described as parasexual. The parasexual cycle (Fig. 34.14) is not in fact a coordinated cycle but rather a series of four events. First, two hyphae fuse, forming a heterokaryotic cell. Then, karyogamy produces a diploid nucleus from two genetically distinct haploid nuclei—so far, a familiar pattern. Next, however, during mitosis, crossing over may occur between the two sets of chromosomes. Crossing over is common during meiosis (Chapter 11) but rare during mitosis. The haploid chromosome number is restored not by meiotic division but by the progressive loss of chromosomes. Like sexual reproduction, these processes produce novel gene combinations. The key difference between parasexuality and sexual reproduction is that parasexual species do not undergo meiosis.
Entirely asexual species of fungi posed a problem for early taxonomists because these species lack many of the morphological characters such as fruiting bodies or sporangium-bearing stalks previously used to define different groups. With the advent of DNA sequence data, it has become increasingly clear that many asexual species are closely related to sexual forms, indicating that their evolutionary history as asexual species is not long. A major exception are the Glomeromycota, for which fossils as old as 450 million years have been reported.