34.3 DIVERSITY
When Aristotle classified the living world into animals and plants, he grouped fungi with plants because of their lack of motility. As discussed in Chapter 27, molecular sequence comparisons now make it clear that fungi are actually related more closely to animals, such that it would not be amiss to refer to mushrooms and molds as our “cousins.”
Fungi share a number of features with animals: Their motile cells, when present, have a single flagellum attached to their posterior ends; fungi and many animals synthesize chitin; and fungi store energy as glycogen, as do animals. Nevertheless, the last common ancestor of fungi and animals was a single-celled microorganism that lived in aquatic environments about 1 billion years ago. Multicellularity evolved independently in fungi and animals (Chapter 28), and so the fungi have many features that are unique in the biological world.
34.3.1 Fungi are highly diverse.
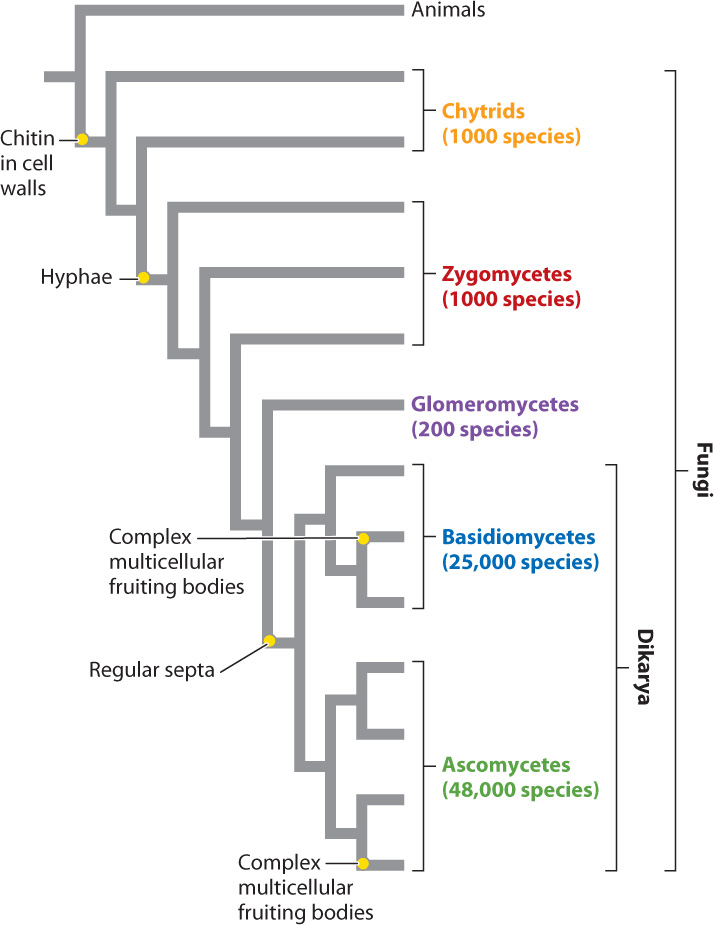
By any measure, fungi are diverse, although just how diverse is not known. About 75,000 species have been formally described, but estimates of true fungal diversity run as high as 5 million species. Among eukaryotes, the only organisms more diverse than fungi are the animals. The availability of DNA sequence data has greatly advanced our understanding of phylogenetic relationships within the fungi, which are shown in Fig. 34.15. The tree clearly shows how the characters present in familiar mushrooms accumulated through the course of evolution: first chitinous cell walls, then hyphae, then regularly placed septa, and finally the complex multicellular reproductive bodies we call mushrooms. Motile single cells are found only in the most ancient lineages, while dikaryotic cells and complexity of form are found only on the most recent branches.
The evolutionary history of fungi can be viewed as the transition from a flagellated ancestral form that lived in aquatic environments to soil-dwelling forms in which hyphae divided by septa developed complex morphologies visible to the naked eye. As we saw in the preceding section, another evolutionary trend is the progressive separation in time between plasmogamy and karyogamy in fungal life cycles. Many of the distinguishing features of fungi evolved as adaptations to the challenges faced by nonmotile, absorptive heterotrophs on land.
We also see that the numbers of species are not spread evenly across the phylogeny. Instead, more ancient lineages include less than 2% of known species, while the two dikaryotic groups include more than 98% of known fungi. Clearly, dikaryotic fungi are well adapted to many different habitats, including other organisms, both living and dead.
Question Quick Check 3
1q0QnzDvfGNqnKTG/fmkLVBTLPJ6mzOkxabmu7/WevFGh6VQrWI7uDFr/5K7Ll53S0xRMrSr1mcN29uLPLgaWysgg1WbBCTkCWuIQ0waC8+L2ObpBseGD/YvHTeTa6rrprTRyTezQ0ViG55ZKrVQwj2dHn4VHKm/L2njXQgLUq1sx2R6XyJLxXNlrY9pjqOXmLyRQnM0Dy/feveW34.3.2 Fungi evolved from aquatic, unicellular, and flagellated ancestors.
Fungi are opisthokonts, members of the eukaryotic superkingdom that includes animals (Chapter 27). Among fungi, the Chytridiomycota (commonly referred to as chytrids) lie at the base of the phylogenetic tree. This is a small group of about 1000 species found in aquatic or moist environments (Fig. 34.16). Many chytrids are single cells with walls of chitin. They may also form short multinucleate structures, but they lack the well-defined hyphae characteristic of other fungi. Chytrids, therefore, do not form a true mycelium, although in some species elongated cellular outgrowths called rhizoids penetrate into organic substrates. Rhizoids anchor the organism in place and absorb food molecules. Chytrids generally disperse by flagellated spores. Sexual reproduction appears to be rare, but there is substantial life-cycle diversity within this group. Chytrids lack a heterokaryotic stage but form flagellated gametes that swim through their aqueous environment.
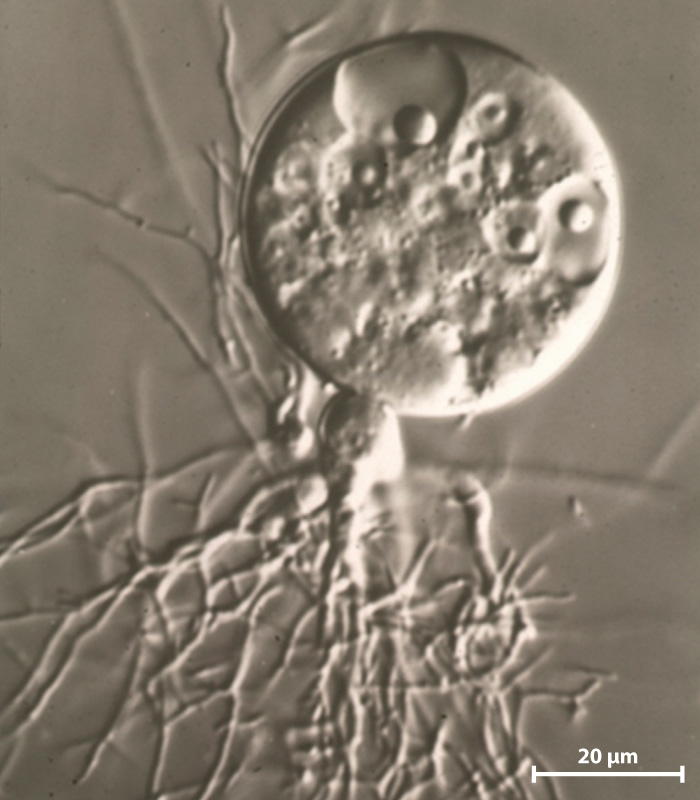
Most chytrids are decomposers, and a few of them are pathogenic. Notably, the chytrid Batrachochytrium dendrobatidis is associated with widespread mortality in amphibian populations (Chapter 48). Molecular clock analysis estimates that chytrids began to diversify 700 to 800 million years ago.
34.3.3 Zygomycetes produce hyphae undivided by septa.
Moving through the phylogenetic tree, we next encounter several groups traditionally united as the Zygomycota, or zygomycetes (see Fig. 34.15), which share a number of traits.
Collectively, zygomycetes make up less than 1% of known fungal diversity. Some are decomposers, specializing on dead leaves, animal feces, and food—there are probably zygomycetes in your kitchen. Others live on and in plants, animals, and even other fungi. Zygomycetes have many typical fungal traits, including the growth of a mycelium and the production of aerial spores. These traits may have evolved as adaptations for finding food and dispersing on land. The loss of flagellated spores may also reflect adaptation for life on land. Unlike more complex fungi, zygomycetes do not form regular septa along their hyphae, nor do they produce multicellular fruiting bodies.
Earlier, we introduced the black bread mold Rhizopus, which is a zygomycete (see Fig. 34.10b). Rhizopus and its relatives are specialists on substrates containing abundant, easy-to-digest carbon compounds, such as bread, ripe fruits, and the dung of herbivorous animals. These fungi consume their substrates rapidly, and once their meal is finished, they release large numbers of aerial spores to locate another food source.
Sexual reproduction occurs when two compatible hyphal tips fuse to form a thick-walled structure containing many nuclei of each mating type. Karyogamy and meiosis are followed by germination of the haploid cell to form an elevated stalk. Each stalk develops a sporangium that contains spores produced asexually by mitotic cell division. If you look closely at a bread mold, you can see the white filamentous hyphae and small black sporangia on slender stalks. Each sporangium can produce as many as 100,000 spores that are dispersed by the wind, explaining how these organisms seem to get everywhere.

Although zygomycete fungi do not form multicellular fruiting bodies, some have evolved truly spectacular means of dispersal. Pilobilus, which consumes the dung of herbivorous animals, has a life cycle similar to Rhizopus except that instead of releasing individual spores, it forcibly ejects the entire sporangium (Fig. 34.17). Turgor pressure generated in the supporting stalk propels the sporangia as far as 2 m. Light-sensitive pigments in the stalk’s hyphae control the orientation of this water cannon, ensuring that the spores have the best chance of escaping the dung pile and landing on vegetation that is attractive to grazing herbivores. Feeding herbivores disperse the spores further, while supplying them with fresh dung for food. The remarkable dispersal of Pilobilus sporangia benefits other species, as well: Parasitic nematode worms hitch a ride on the sporangia to find a new host.
34.3.4 Glomeromycetes form endomycorrhizae.
The Glomeromycota, or glomeromycetes, are a monophyletic group of apparently low diversity (there are about 200 described species) but tremendous ecological importance. All known glomeromycetes occur in association with plant roots. Most famous are those that form endomycorrhizae. Molecular clock estimates suggest that glomeromycetes diverged from other fungi 500 to 600 million years ago, before the evolution of vascular plants. Establishment of glomerophyte-root symbiosis changed the course of evolution for both participants. Today, most vascular plant species harbor endomycorrhizae, dramatically increasing nutrient uptake from the soil.
Glomeromycetes are hard to study because they cannot be grown independently of their plant partners, and so many unanswered questions remain. For example, no evidence of sexual reproduction has ever been found in this group. Yet genetic studies of glomeromycete populations show some genetic diversity, which is thought to result from parasexual processes (see Fig. 34.14). Glomerocytes produce extremely large (0.1 to 0.5 mm diameter) multinucleate spores asexually. These large spores can persist in soils until they come into contact with an uninfected root.
34.3.5 The Dikarya produce regular septa during mitosis.
The final major node, or branching point, in the fungal phylogeny marks the origin of the Dikarya, a vast group that includes about 98% of all described fungal species. A key feature of the dikaryotic fungi is that every mitotic division is accompanied by the formation of a new septum. This innovation allows these fungi to control the number of nuclei within each cell and thus to proliferate dikaryotic cells. The Dikarya include all the edible mushrooms, the yeast species used in the production of beer, bread, and cheese, the major wood-rotting fungi, and pathogens of both crops and humans.
The Dikarya contain two monophyletic subgroups (see Fig. 34.15), each named for the shape of the cell in which the key reproductive processes of nuclear fusion (karyogamy) and meiosis take place. The Ascomycota, or ascomycetes, are sometimes called sac fungi because nuclear fusion and meiosis take place in an elongated saclike cell called an ascus (askos is Greek for “leather bag”). The Basidiomycota, or basidiomycetes, are popularly known as club fungi because the nuclear fusion and meiosis take place in a club-shaped cell called a basidium. (“Basidium” is derived from the Greek basis, which means “base” or “pedestal” in Greek; the reference is to the position of sexually produced spores at the tips of this club-shaped body.)
Although ascomycetes and basidiomycetes share the same basic life cycle, they diverged from each other as much as 600 to 500 million years ago and evolved complex multicellular structures independently of each other. The morel in the supermarket is an ascomycete, whereas the shiitake mushroom next to it is a basidiomycete (Fig. 34.18). In addition to asci and basidia, the two groups differ in several other features. For example, the septal pores that connect adjacent cells within hyphae are distinct in the two groups, as are the ways that cells can plug the pores to minimize damage to a mycelium. Also, when ascomycetes reproduce, mating cells form and their nuclei fuse in relatively close succession. Thus, the heterokaryotic stage is brief. In contrast, in the basidiomycetes, the nuclei of two fused cells may remain separate for a long time. In this case, hyphae containing both types of nuclei (dikaryotic hyphae) can continue to absorb nutrients from the substrate for a while before nuclear fusion occurs.

34.3.6 Ascomycetes are the most diverse group of fungi.
Ascomycetes make up 64% of all known fungal species. They include important wood-rotting fungi, many ectomychorrizal species, and significant pathogens of both animals and plants. Ascomycetes form the fungal partner in most lichens— approximately 40% of all ascomycete species occur in lichens. Ascomycetes loom large in human history, contributing the baker’s and brewer’s yeasts used to make bread and beer, the antibiotic penicillin, and model systems employed in laboratory investigations of eukaryotic cell biology and genetics. Ascomycetes are used to produce soy sauce, sake, rice vinegar, and miso, and to transform milk into Brie, Camembert, and Roquefort cheeses. Athlete’s foot and other skin infections are caused by ascomycetes, as are many more serious fungal diseases.
Ascomycetes include both species that form multicellular fruiting bodies and ones that form unicellular yeasts. A remarkable feature of ascomycete fruiting bodies is that they contain both dikaryotic and haploid cells. The proliferation of dikaryotic cells leads to the production of many asci, while the growth of haploid cells contributes to the bulk of the fruiting body. Two ascomycete lineages consist largely of yeasts. The earliest branching members in these groups, however, produce hyphae. Thus, the unicellular nature of yeasts is a derived feature evolved through loss of hyphae and is not an ancestral condition.
Fig. 34.19 illustrates the life cycle of a common ascomycete, the brown cup fungus. In ascomycetes, meiosis is followed by a single round of mitosis resulting in asci that contain eight haploid spores. In many ascomycetes, fruiting bodies elevate the asci on one or more cup-shaped surfaces, from where they are easily caught and carried away by wind. When mature, the spores are ejected from the top of the asci, expelled by turgor pressure.
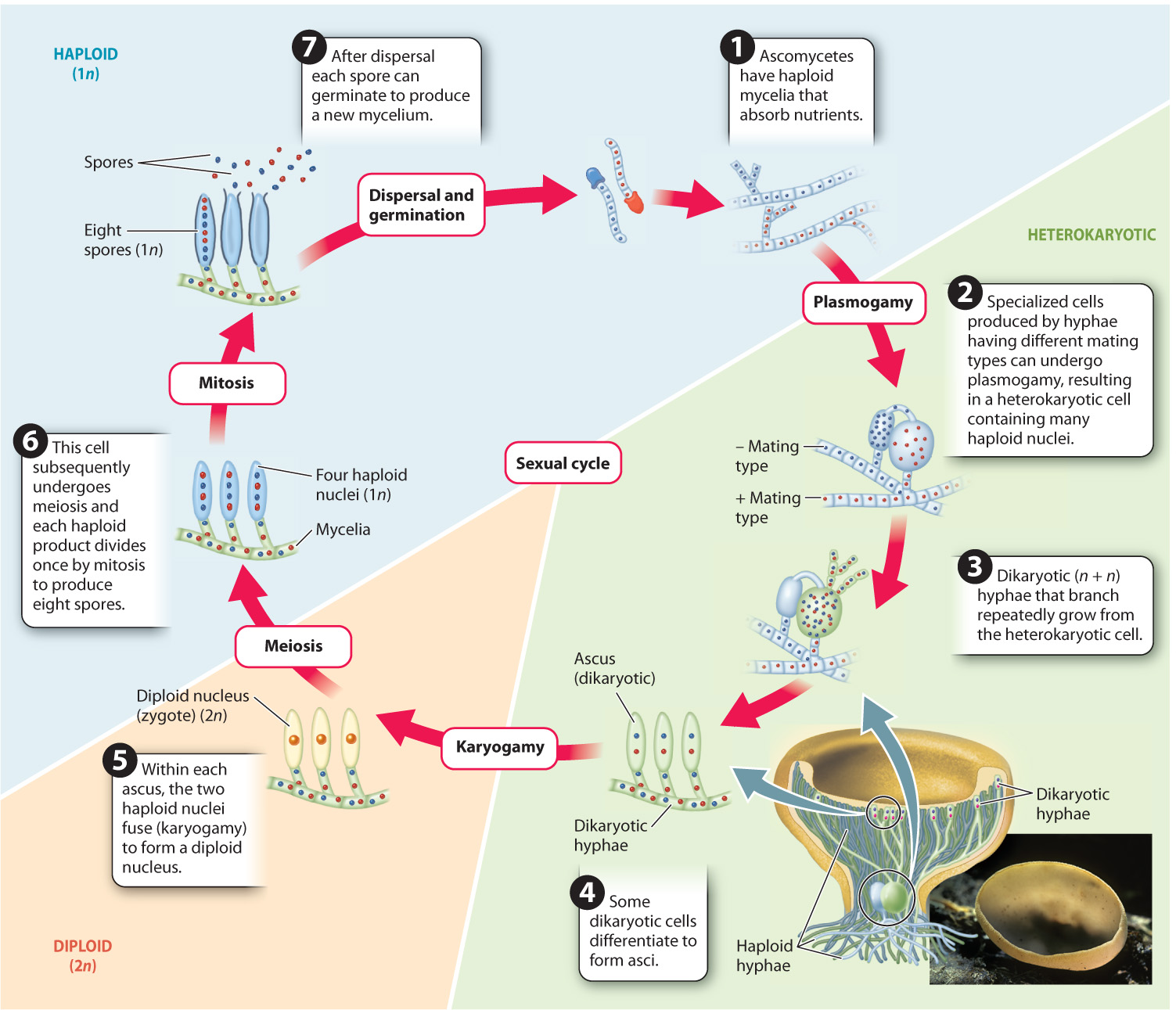
In the fruiting bodies of some ascomycetes, the asci are completely enclosed by a layer of tissue and thus must be dispersed by other organisms rather than by the wind. The truffles prized in cooking provide an example. The edible truffle is the fruiting body of an ascomycete that grows as an ectomycorrhizal fungus on tree roots. Not only do truffles encase their spores in protective tissues, but they also develop underground. How, then, can their spores be dispersed? Developing truffles release androstenol, a hormone also produced by boars before mating. The hormone attracts female pigs, which unearth and consume the fruiting body. The spores pass through the pig’s digestive tract without damage and are released into the environment in feces, thereby dispersing the spores.
Another ascomycete is thought to have played a role in the events that unfolded in Salem, Massachusetts, in 1692. That year, several girls came down with an unknown illness manifested by delirium, hallucinations, convulsions, and a crawling sensation on the skin. The girls were thought to be bewitched, and subsequent accusations led to the infamous Salem witch trials. They were executed for witchcraft, but today many scholars prefer a simpler diagnosis for the illness: poisoning by the ascomycete fungus ergot. Ergot is a common pathogen of rye and related grasses, and ingestion of contaminated plants can lead to the symptoms reported in Salem. Like many defensive compounds produced by plants, alkaloid molecules produced by ergot and their derivatives are used in medicine in low doses, such as in the treatment of migraines.
Ascomycetes are even known for producing so-called zombie ants, a topic explored in Fig. 34.20.
FIG. 34.20: Can a fungus influence the behavior of an ant?
BACKGROUND A curious death ritual unfolds in the rain forest of Thailand. Spores of the ascomycete fungus Ophiocordyceps infect Camponotus leonardi ants, growing hyphae inside their bodies. The fungus eventually kills the ant, and fruiting bodies emerge from the dead ant’s head to disperse spores that begin the life cycle anew. C. leonardi nests and forages for food high in the forest canopy. Infected ants, however, undergo convulsions that cause them to fall to the forest floor. The infected ants wander erratically and are unable to climb more than a few meters above the ground before convulsions make them fall again. In their final act, the ants bite into leaves and die. The fungus is then able to complete its life cycle within the humid forest understory. Is the ants’ behavior induced by the fungi?

An infected ant attached to a leaf by having bitten into it in a “death grip.”
HYPOTHESIS Parasitic fungi manipulate ant behavior to complete their own reproductive cycle.
OBSERVATIONS Infected and uninfected ants were observed for many hours and behavioral events were recorded.
RESULTS Infected ants have repeated convulsions, but uninfected ants show no such behavior. Transitions from erratic wandering to a “death grip,” in which the ant bites into a leaf, occurred at about the same time. Dissections showed that the ants’ death grip results from jaw-muscle wasting caused by the fungus.
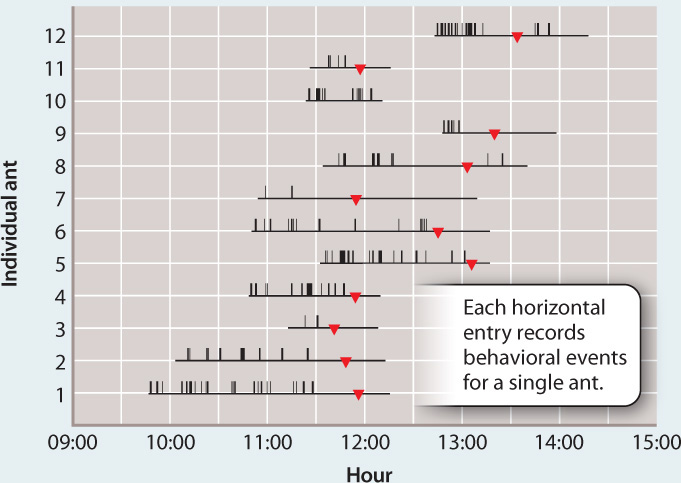
CONCLUSION The fungi change the behavior of infected ants, inducing a stereotypical behavior that facilitates completion of the fungus’ life cycle. The molecular basis of this manipulation is not yet known.
FOLLOW-UP WORK The zombie-like behavior induced by the fungus is an example of what has been called the extended phenotype: the idea that a phenotype should include the effects that an organism has on its environment—in this case, its effects on host behavior.
SOURCE Hughes, D. P., S. B. Andersen, N. L. Hywel-Jones, W. Himaman, J. Billen, and J. J. Boomsma. 2011. “Behavioral Mechanisms and Morphological Symptoms of Zombie Ants Dying from Fungal Infections.” BMC Ecology 1 1:13-22.
34.3.7 Basidiomycetes include smuts, rusts, and mushrooms.

The basidiomycetes, which make up 34% of all described fungal species, include three major groups (Fig. 34.21). Two of these—the smuts and rusts—consist primarily of plant pathogens. Smut fungi, which infect the reproductive tissues of grasses and related plants, take their name from the black sooty spores that they produce. Ustilago maydis, the corn smut, turns developing corn kernels into soft gray masses that are a culinary delicacy in Mexico. Because smuts infect seeds, their spread through crops is magnified by the harvest, storage, and eventual sowing of seeds. Before the introduction of chemical seed treatments in the 1930s, infection by Tilletia (the stinking smut) commonly caused losses of up to 50% of the wheat harvest. Smuts remain a problem in parts of the world where untreated seeds are still planted.
Rusts can also have profound impacts on plant function and development. For example, the rust Puccinia monoica alters leaf development in its host plant Arabis, resulting in a pseudoflower that attracts insects by visual and olfactory cues as well as nectar rewards. These pseudopollinators transport spores, thus enhancing outcrossing as well as dispersal of the fungi but provide no service to the plant. We discuss the biology of rusts in greater detail in the next section when we examine Ug99, a highly virulent wheat rust first reported in Uganda in 1999.
The third basidiomycete group is characterized by the formation of multicellular fruiting bodies, including the iconic toadstools with their central stalk and umbrella-like cap. However, this group also includes the diverse shapes seen in stinkhorns, puffballs, and bracket fungi (Fig. 34.22).

Fig. 34.23 shows the life cycle of a typical basidiomycete mushroom. The life cycle is similar to that of ascomycetes (see Fig. 34.19), except that the fruiting body is made up entirely of dikaryotic hyphae instead of a combination of dikaryotic and haploid hyphae as in ascomycetes. In addition, nuclear fusion (karyogamy) takes place in a specialized cell called a basidium rather than an ascus. Finally, the haploid products of meiosis (the spores) do not undergo mitosis, so four spores are produced from each basidium rather than eight spores from each ascus.
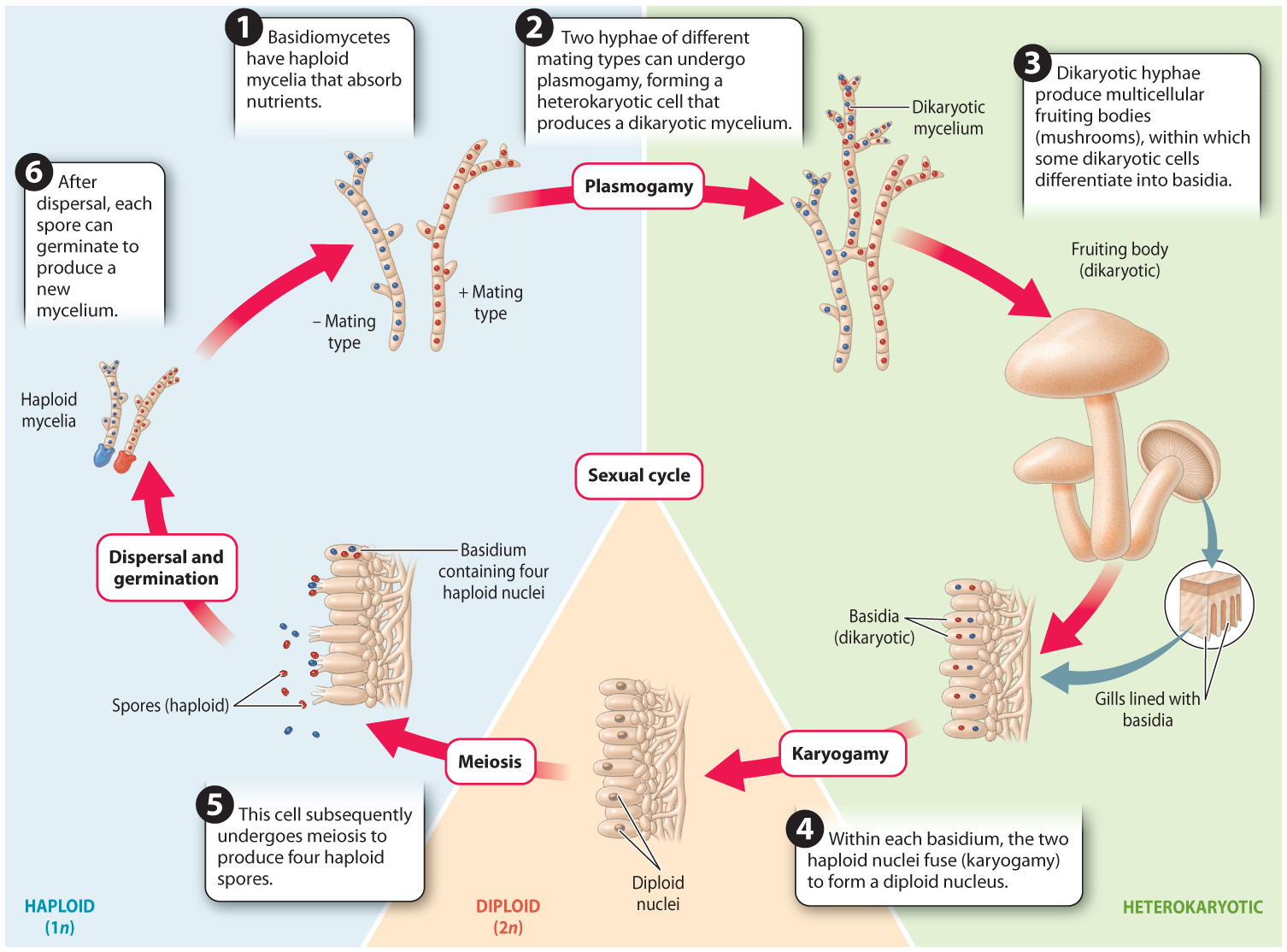
Question Quick Check 4
DbTr/jN/QPyNPdejW1FOYLJmdTCPh4iC47uWxPHi/oauK5OZFpCpjAVXT0WFV4W6MxMLcJZVIP0Bn4vBred/+mbmpuh5+z6bcuyyAA==Most species in this group live by forming ectomycorrhizal associations or by decomposing wood and other substrates. The fruiting bodies that we see elevated above a rotting log or the soil are connected to extensive mycelia that provide resources for fruiting-body development.
Elevation alone does not ensure dispersal because the spores must penetrate a boundary layer of air that surrounds the fruiting body. Many basidiomycetes depend on surface tension to catapult their spores through the air. The placement of their four spores on the ends of short stalks is the key to this mechanism. Both the spores and the supporting basidial cell actively secrete solutes that cause water to condense on their surfaces. At first, the droplets are independent, but as they grow they eventually come into contact with one another, and at this point the high surface tension of water causes them to merge into a single, smooth droplet. This change in shape shifts the center of mass of the water with such force that it catapults the spore into the air.
Basidiomycetes that form conspicuous fruiting bodies have diverse mechanisms for dispersing their spores. For example, puffballs function like bellows: The impact of raindrops forces the loose, dry spores out of a small hole at the tip of the ball (see Fig. 34.10a). Bird’s nest fungi function like splash cups: In this case, raindrops displace groups of spores (the “eggs” in the nest) onto nearby vegetation. Herbivores consume the spores when they feed on the vegetation and transport them to a new dung pile. Stinkhorns produce spores in a stinky, sticky mass that attracts insects, which then carry off spores that stick to their legs and body.
34.3.8 How do fungi threaten global wheat production?
Case 6 Agriculture: Feeding a Growing Population
Throughout much of human history, crops have been vulnerable to infection by Puccinia graminis, the black stem rust of wheat. P. graminis infects wheat leaves and stems through their stomata and then extends within the plant’s living tissues to fuel its own growth. Rust-colored pustules erupt along the stem and then release a vast number of spores, leaving the host plant to wither and die. Crop losses from P. graminis can be devastating. In the past, both the United States and the Soviet Union tried to develop black stem rust as a biological weapon. The U.S. version was cluster bombs (since destroyed) containing turkey feathers smeared with rust spores, produced with the aim of disrupting crop yields in enemy countries.
Many rusts have a life history in which they alternate between different host species. For example, to complete its life cycle, P. graminis must infect wheat first and then barberry (Berberis species). After a particularly severe outbreak of wheat rust during World War I, in which crop losses equaled one-third of total U.S. wheat consumption, a major program of barberry eradication was started to bring stem rusts under control. This program reduced the threat, but did not totally eliminate it.
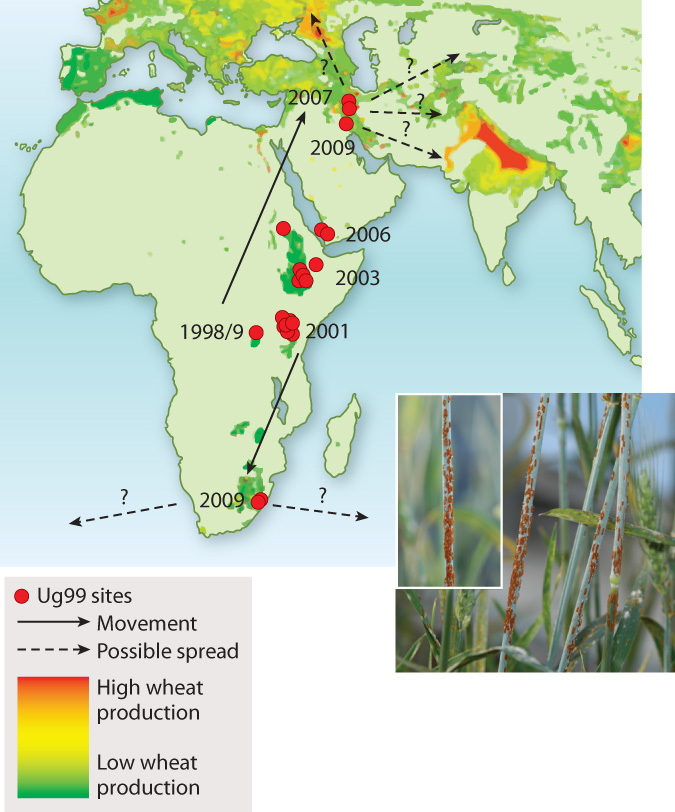
The Green Revolution seemed to give growers a decisive advantage over stem rust. During World War II, Norman Borlaug, whose original training was in plant pathology, was sent to Mexico by the Rockefeller Foundation to help combat crop losses due to stem rust. Borlaug’s breeding efforts led to resistant wheat varieties. Much of this resistance could be attributed to variation in a small number of genes. Stem rusts were apparently vanquished—that is, until the appearance of Ug99, a wheat stem rust first identified in Uganda in 1999 (hence the name). Ug99 is capable of defeating all major resistance genes. Given that wheat accounts for 20% of daily global calorie consumption by humans and that 90% of cultivated wheat has little or no resistance to Ug99, the appearance of this resistant fungus is cause for major concern. At present, Ug99 shows every sign of spreading (Fig. 34.24)—not surprising given that a single hectare of infected wheat produces upward of a billion spores. It currently devastates wheat production in Kenya and has been found in Yemen (2006), Iran (2007), and South Africa (2009). As you read this, Ug99 is poised to reach the major wheat growing regions of Turkey and South Asia. Even more rapid spread is possible if spores accidentally lodge on cargo or airline passengers.
Just before his death in 2009, Norman Borlaug urged the world to take this threat seriously. Today, plant breeders are actively searching ancestral wheat varieties for genetic sources of resistance to Ug99, while other scientists are trying to understand how plants defend themselves against rusts and what makes Ug99 so virulent. The resurgence of P. graminis as a serious threat to world food production underscores the importance of safeguarding the genetic diversity of agricultural species so that the inevitable evolutionary battles with pathogenic species may be waged successfully.