37.1 MUSCLES: BIOLOGICAL MOTORS THAT GENERATE FORCE AND PRODUCE MOVEMENT

We rely on our muscles for all kinds of movement. Muscles function as biological motors within the body because they generate force and produce movement. Some of these movements are obvious—running, climbing up stairs, raising your hand, playing the piano. We are less aware of others—moving food through the digestive tract, breathing in and out, pumping blood through the body. A muscle’s ability to produce movement depends on a combination of electrically excitable muscle cells and contractile proteins within these cells that can be activated by the nervous system.
The contractile machinery of muscles is ancient. The proteins underlying muscle contraction are found in eukaryotes that lived more than 1 billion years ago, and the first muscle fibers common to all animals are found in cnidarians (jellyfish and sea anemones), which lived at least 600 million years ago. Thus, basic features of muscle organization and function are conserved across the vast diversity of eukaryotic life. As we will discuss, the geometry and organization of these proteins largely determines how muscles contract to produce force and movement.
37.1.1 Muscles can be striated or smooth.
Muscle cells, or fibers, produce forces within an animal’s body, as well as exert forces on the environment. Despite their diversity, all muscle cells produce force by a similar mechanism. Skeletal muscle fibers produce forces by shortening, thus pulling on bones of the skeleton. Muscles pull on the bones of your leg and foot to swing them forward when you kick a ball. Muscle fibers in the heart contract to pump blood. Still other muscle fibers control blood flow through arteries by contracting to change an artery’s cross-sectional shape, or are used for movements involved in digestion and reproduction.
Muscles can shorten very quickly, and they can produce large forces for their weight. The forces they produce can be many times an animal’s body weight, allowing the animal to accelerate quickly. Some small muscles contract extremely quickly: The muscles of many flying insects contract 200 to 500 times per second. Even in vertebrate animals, muscles can contract as many as 50 to 100 times per second. Other muscles, such as the closing muscle of a clam, contract slowly, but do so for prolonged periods without tiring.
37-2
All muscles contain contractile proteins that enable them to shorten and produce force, but they are divided into two broad groups on the basis of their function and appearance under a light microscope (Fig. 37.2). As their name suggests, striated muscles appear striped under a light microscope. They include skeletal muscles (Fig. 37.2a), which connect to the body skeleton to move the animal’s limbs and torso, and cardiac muscle (Fig. 37.2b), which contracts to pump blood. In contrast, smooth muscles appear uniform under the light microscope (Fig. 37.2c). They are found in the walls of arteries to regulate blood flow, in the respiratory system to control airflow, and in the digestive and excretory systems to help transport food and waste products. Smooth muscles contract slowly compared to cardiac and skeletal muscles.
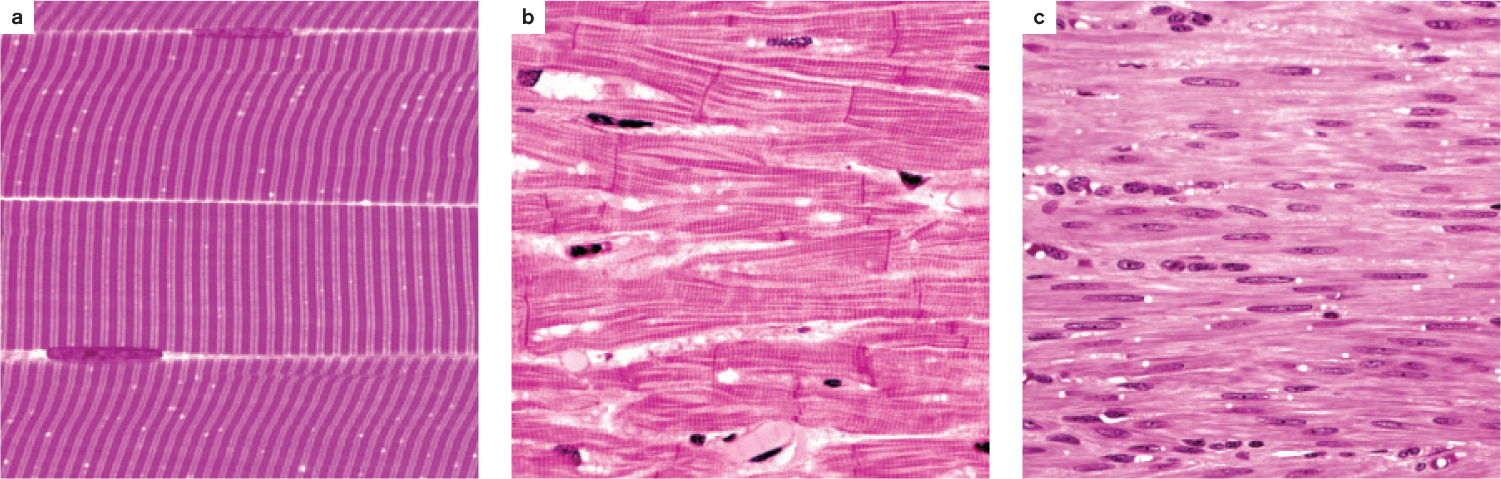
Striated and smooth muscles use the same sets of muscle proteins—actin and myosin (Chapter 10)—to contract and generate force (force is mass times acceleration, and in this case is the ability to accelerate the motion of the body). These proteins are organized into thin threads called filaments that interact with one another to cause muscles to shorten.
The actin and myosin filaments in striated muscles are arranged in a regularly repeating pattern, producing the striations that can be observed under the microscope. In contrast, the organization of actin and myosin filaments in smooth muscles is irregular, giving these muscles a smooth appearance when viewed under the light microscope.
37.1.2 Skeletal muscle fibers are organized into repeating contractile units called sarcomeres.
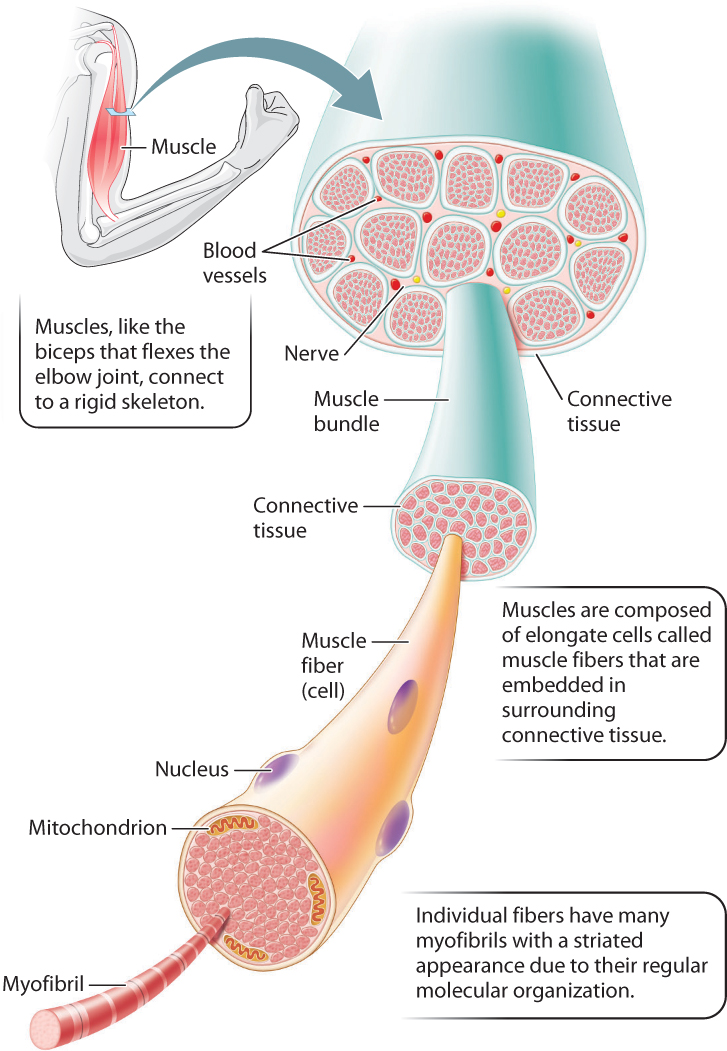
The visible bands in skeletal muscles turn out to be an important clue to the mechanism used to produce muscle contractions. Whole muscles are made up of parallel bundles of individual muscle fibers (Fig. 37.3). Each muscle fiber is an elongated single cell that can be up to 20 cm long. It has several nuclei and therefore is described as multinucleated. The cell’s multinucleated organization means that gene expression must be coordinated among all nuclei in the muscle fiber. Coordinated gene expression is necessary when endurance training increases the cell’s rate of ATP production, so that the entire cell contracts uniformly. Each muscle fiber contains hundreds of long rodlike structures called myofibrils that contain parallel arrays of the actin and myosin filaments that cause a muscle to contract.
37-3
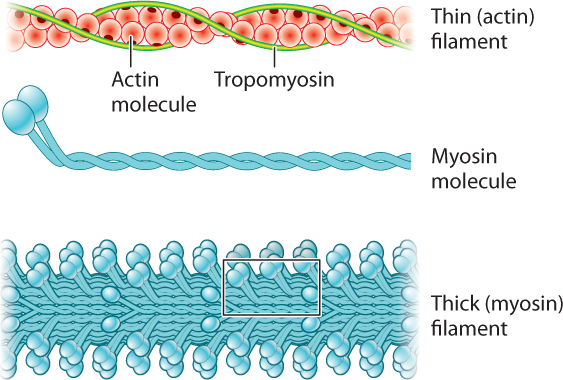
Let’s consider the structural organization of myosin and actin filaments in more detail (Fig. 37.4). Each myosin molecule consists of two long polypeptide chains coiled together, each ending with a globular head. Consequently, the myosin molecule resembles a double-headed golf club. The myosin molecules are arranged in parallel to form a thick filament, with the numerous myosin heads extending out from their flexible necks along the myosin filament. Two helically arranged actin filaments are twisted together to form a thin filament. The protein tropomyosin runs in the grooves formed by the actin helices.
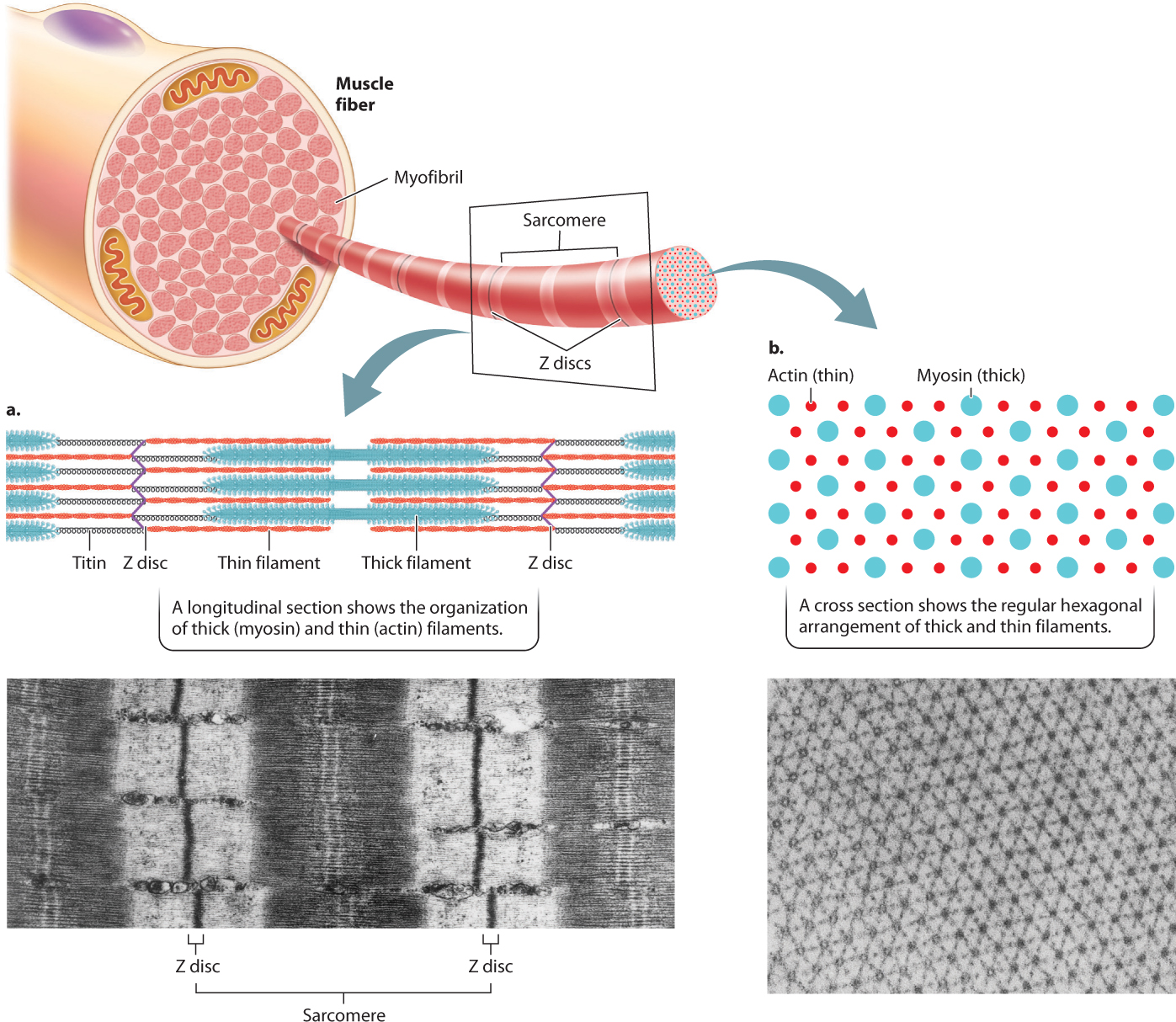
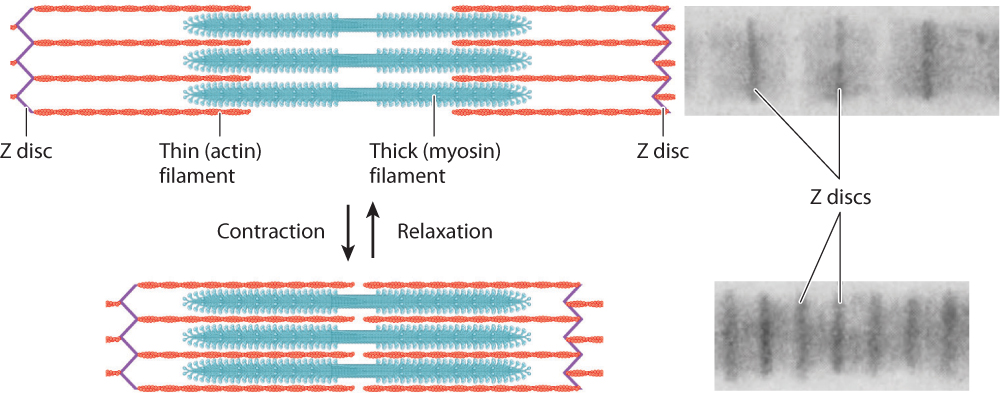
The thin filaments are attached to protein backbones called Z discs that are regularly spaced along the length of the myofibril (Fig. 37.5a). The region from one Z disc to the next, called the sarcomere, is the basic contractile unit of a muscle. The sarcomeres are repeating contractile units arranged in series along the length of the muscle fiber. The shortening of thousands of sarcomeres along a fiber leads to shortening and contraction of a muscle.
Sets of actin thin filaments extend from both sides of the Z discs toward the midline of the sarcomere (Fig. 37.5a). In the middle of the sarcomere, not directly contacting the Z discs, are myosin thick filaments. The thin filaments overlap with the myosin thick filaments, forming two regions of overlap within a sarcomere. Toward either end of the sarcomere, as well as in the middle, are regions where the actin and myosin filaments do not overlap. These regions of non-overlap are lighter in color than the regions that overlap, which appear dark under an electron microscope. A third large protein, called titin, links the myosin filaments to the Z discs at the ends of the sarcomere. Titin is believed to help with assembly and to protect the sarcomeres from being overstretched, thus contributing to muscle elasticity.
As we have seen, the regular pattern of actin and myosin filaments within sarcomeres along the length of the fiber gives skeletal muscles their striated appearance (Fig. 37.5a). In cross section, thick and thin filaments are arranged in a hexagonal lattice that allows each thick filament to interact and slide with respect to six adjacent thin filaments (Fig. 37.5b). The precise geometry of thin and thick filaments is critical to their interaction, discussed on the following page.
37-4
37.1.3 Muscles contract by the sliding of myosin and actin filaments.
By using high-resolution light microscopy, two independent teams (physiologists Hugh Huxley and Jean Hanson in the United States and Andrew Huxley and Rolf Niedergerke in England) were able to quantify the changing banding pattern of sarcomeres when rabbit or frog myofibrils were stimulated to contract to different lengths. Their results led them to hypothesize that muscles produce force and change length by the sliding of actin filaments relative to myosin filaments. Their theory is known as the sliding filament model of muscle contraction.
The banding patterns of sarcomeres reveal the extent of overlap between actin and myosin filaments. When myofibrils contracted to short lengths, the sarcomeres were observed to have increased actin–myosin overlap. When myofibrils were stretched to longer lengths, actin–myosin overlap decreased (Fig. 37.6). However, the lengths of the myosin and actin filaments never varied. Consequently, nearly all of the length change during a muscle contraction results from the sliding of actin filaments with respect to myosin filaments within individual sarcomeres. The length change of the whole muscle fiber is therefore a sum of the fractions by which each sarcomere shortens along the fiber’s length.
A muscle’s ability to generate force and change length is largely determined by the properties of its sarcomeres. Whereas sarcomere length is quite uniform in vertebrate animals (about 2.3 µm at rest), it is variable in invertebrate animals (ranging from as short as 1.3 µm to as long as 43 µm). Longer sarcomeres allow a greater degree of shortening. Thus, length changes are more limited in vertebrate muscles compared with some invertebrate muscles that have long sarcomeres. For example, when the muscles in an octopus tentacle contract, they can produce large motions of the tentacle. However, because they have shorter sarcomeres, vertebrate muscles can shorten to produce movements more quickly.
37-5
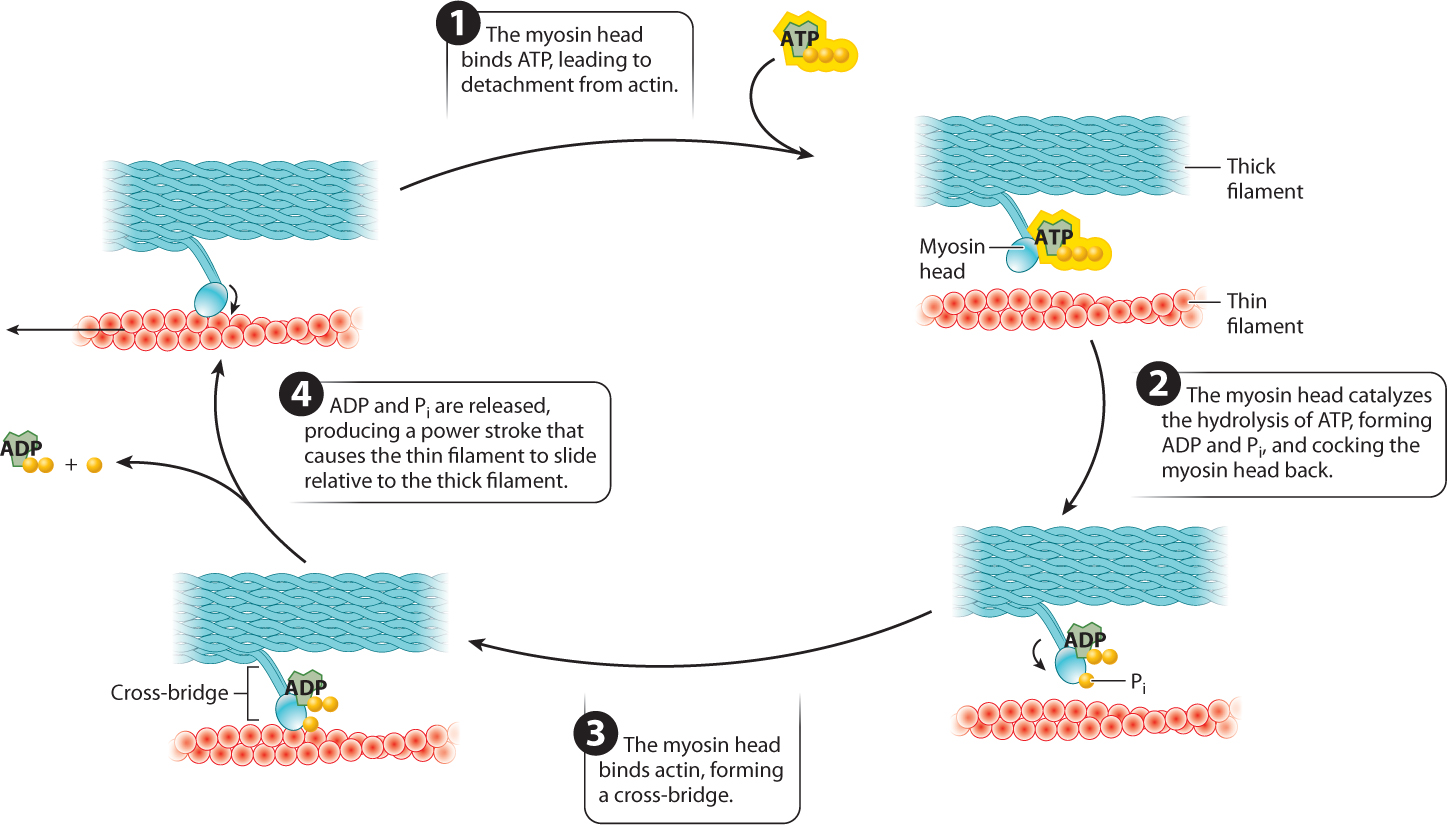
Interactions between the myosin and actin filaments are what cause a muscle fiber to shorten and produce force. Along the myosin filament, the two heads of myosin molecules bind to actin at specific sites to form cross-bridges between the myosin and actin filaments. The myosin filaments pull the actin filaments toward each other by means of the cross-bridges they form with actin filaments. The key to making the filaments slide relative to each other is the ability of the myosin head to undergo a conformational change and therefore pivot back and forth. The myosin head attaches to the actin filament and pivots forward, sliding the actin filament toward the middle of the sarcomere. The myosin head then detaches from the actin filament, cocks back, reattaches, and the cycle repeats. The movement of the myosin head is powered by ATP. Together, these events describe the cross-bridge cycle.
The cross-bridge cycle is just that—a cycle (Fig. 37.7). Let’s look at it in more detail:
- The myosin head binds ATP. Binding of ATP allows the myosin head to detach from actin.
- The myosin head hydrolyzes ATP to ADP and inorganic phosphate (Pi). Hydrolysis of ATP results in a conformational change in which the myosin head is cocked back. ADP and Pi remain bound to the myosin head. Because ADP and Pi are bound rather than released, the myosin head is in a high-energy state.
- The myosin head then binds actin, forming a cross-bridge.
- When the myosin head binds actin, the myosin head releases ADP and Pi. The result is another conformational change in the myosin head, called the power stroke. During the power stroke, the myosin head pivots forward and generates a force, causing the myosin and actin filaments to slide relative to each other over a distance of approximately 7 nm. The power stroke pulls the actin filaments toward the sarcomere midline.
37-6
The myosin head then binds a new ATP molecule (step 1 again), allowing it to detach from actin, and the cycle repeats. After detachment, the myosin head again hydrolyzes ATP, allowing it to return to its original conformation and bind to a new site farther along the actin filament. With the release of ADP and Pi, the myosin head undergoes another step of force generation and movement.
Individual muscle contractions are the result of many successive cycles of cross-bridge formation and detachment, during which the chemical energy released by ATP hydrolysis is converted into mechanical work (the product of force and the distance moved—that is, the amount of shortening). Muscle fibers that contract especially quickly, such as those that power insect wing flapping or produce the sound of cicadas and rattlesnake tails, express myosin molecules that have higher rates of ATP hydrolysis, allowing faster rates of cross-bridge cycling, force development, and shortening. Thus, myosin functions as both a structural protein and an enzyme.
Because each thick filament can interact with as many as six actin filaments, at any one time numerous cross-bridges anchor the lattice of myosin filaments, while other myosin heads are detached to find new binding sites. When summed over millions of cross-bridges, these molecular events generate the force that shortens the whole muscle.
Question Quick Check 1
HQ52FyUybca7CrmzxJvm6UwSmBhQ/IzzcwekS5DzCWOEOPXSjlsq2IWyhsysModSzJL5kqeDc0IC+aP89mXhJ00ljvZJXuw5RCdB/pItYe51FtefRNrUs9+3KX38X4r7KRRHIQh0YxLTgRzRK56gn1K8Diskk/4oKCtrMOMkPHIpxPPok9ANlvY2Zq/8O0XM9p/B6Qi4A1Uj3ospeXo2bFx8hRSyo91f4YnJ4uqc5ebNlcX9KpwcP9peJYmv5VllgkukBToSgzI=37.1.4 Calcium regulates actin–myosin interaction through excitation–contraction coupling.
We have discussed what makes muscles contract. We next consider when they contract. Striated and smooth muscle fibers are both activated by the nervous system. Whereas vertebrate skeletal muscles are innervated by the somatic nervous system, smooth muscles are innervated by the autonomic nervous system, as are cardiac muscle fibers (Chapter 35).
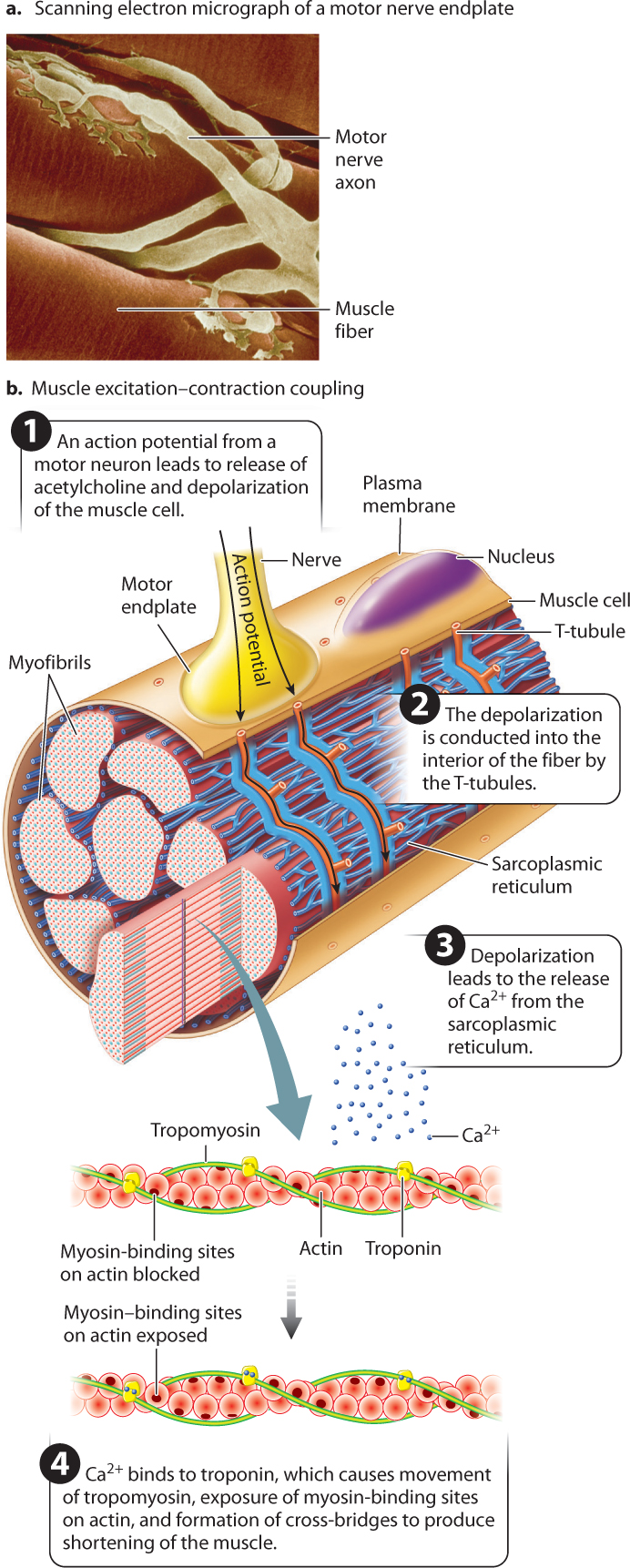
Like nerve cells, muscle fibers are electrically excitable. Skeletal muscle fibers are activated by impulses transmitted by motor nerves to synaptic junctions (Fig. 37.8). Motor neuron axons have branches that allow them to synapse with multiple muscle fibers. When action potentials traveling down a motor neuron arrive at the neuromuscular junction, the neurotransmitter acetylcholine is released into the synaptic cleft. The neurotransmitter binds with receptors on the muscle cell at a region called the motor endplate, triggering the opening of Na+ channels. The resulting influx of Na+ ions in turn initiates a wave of depolarization that passes along the length of the muscle fiber (Chapter 35).
How does membrane depolarization lead to the cross-bridge cycle? Actin and myosin filaments can form cross-bridges only when the myosin-binding sites on actin are exposed. At rest, these binding sites are blocked by the protein tropomyosin. The wave of depolarization in the muscle cell initiates a chain of events that leads to a change in the conformation of a second protein, called troponin, which in turn moves tropomyosin away from these binding sites, allowing cross-bridges between actin and myosin to form and the muscle to contract (Fig. 37.8).
The chain of events that concludes with the exposure of the myosin-binding sites begins at the motor endplate. After the initial membrane depolarization at the motor endplate, the wave of depolarization is conducted inward to deeper regions. You saw in Chapter 5 that eukaryotic cells contain several types of membrane-bound internal organelle. The myofibrils of muscle cells are surrounded by a highly branched membrane-bound organelle called the sarcoplasmic reticulum (SR), a modified form of the endoplasmic reticulum (Fig. 37.8). Depolarization initiated in the plasma membrane is conducted to the SR through a specialized transmission system, formed from invaginations of the plasma membrane, known as the T-tubule system that is in contact with both the muscle cell’s plasma membrane and the SR (Fig. 37.8). When the muscle is at rest, the SR contains a large internal concentration of calcium (Ca2+) ions pumped in by calcium pumps in its membranes.
A muscular contraction is initiated when depolarization of the T-tubules causes the SR to release Ca2+. The Ca2+ diffuses into the myofibrils and binds to troponin, causing the troponin molecule to change shape. This conformational change of troponin, in turn, causes tropomyosin to move, exposing myosin-binding sites along the actin filament. Now myosin cross-bridges can form with actin, producing a contraction. Note that at this stage in the cross-bridge cycle, the myosin head has already hydrolyzed ATP, is bound to ADP and Pi, and is in the cocked-back “ready” position. Binding to actin then allows the power stroke to occur. In this way, contraction is initiated immediately following depolarization and release of Ca2+.
37-7
Thus, Ca2+ release from the SR and binding to the troponin–tropomyosin complex on the actin filament results in excitation–contraction coupling (so called because excitation of the muscle cell is coupled to contraction of the muscle), producing force and movement. Together, these events are the molecular “switch” that causes a muscle to contract. Muscle relaxation follows the end of neural stimulation, when acetylcholine is broken down or reabsorbed and Ca2+ is actively transported back into the sarcoplasmic reticulum, causing tropomyosin molecules to block myosin-binding sites along the actin filaments.
37.1.5 Calmodulin regulates Ca²⁺ activation and relaxation of smooth muscle.
How is the contraction of smooth muscle regulated? The smooth muscle that controls many internal organs can be activated by stimulation from the autonomic nervous system but also responds to stretch of the muscle, local hormones, or other local factors such as pH, oxygen, carbon dioxide, or nitric oxide. For example, smooth muscle in the walls of arteries contracts when stimulated by the release of epinephrine to reduce blood flow to a body region (Chapter 39), and smooth muscle within the mammalian uterus contracts when oxytocin is released to stimulate contractions (Chapter 42). As in skeletal muscle, these initiators trigger the release of Ca2+ from the SR. However, unique to smooth muscle, Ca2+ also enters through voltage-gated and stretch-receptor calcium channels in the cell’s plasma membrane.
Smooth muscle lacks the troponin–tropomyosin mechanism for regulating contraction. Instead, the switch that activates smooth muscle cells is the binding of the protein calmodulin with Ca2+ released from the SR or entering through the cell’s membrane. The calmodulin–Ca2+ complex activates the enzyme myosin kinase, which phosphorylates the smooth muscle myosin heads, causing them to bind actin and begin the cross-bridge cycle. A second enzyme dephosphorylates the myosin heads, disrupting their ability to bind to actin, and the muscle relaxes.
The SR of smooth muscle cells is less extensive and has many fewer calcium pumps than the SR of skeletal muscle cells. As a result, calcium is returned more gradually from the myofibrils back to the SR, or pumped back out through the cell’s membrane, slowing the relaxation of smooth muscle and resulting in longer periods of contraction compared with skeletal muscle fibers.
Question Quick Check 2
BuevLYiDWEOUQalQ6SJ4bJxsAnoAQrsRkzjxWBFGJfl6NrkH+/mU/9ixC6p5zMSvqUcxStYi9wyHjg/6dP3nS0zbRz7+M7TIaw0GwPn4oRlR279Q+OqqyxNHrQaG1eeXQlr7+O2fCm2Nt3uJbhnvQc5wz4OmPy5CwzdPHrLqqvNbF2nF6po8t2cwcggriLM9aVbOj89n5qb90xcfUjfGllG+i3obK/FhOeYc0GG3JqeuuamewM+2TCh0MIlHd6Cnd2XVjk5gjlu7QHWLU+17vmb/RmY6HhqXmzHkWWoMWSP3bCiukLUdnWw42DnVOISt4tZ9D4aZg3PN+fO65S8Kmw==37-8