39.3 OXYGEN TRANSPORT BY HEMOGLOBIN
Once O2 is extracted from the environment and diffuses into the animal, it is transported to respiring cells. Oxygen is transported in the circulation by specialized body fluids—the blood of vertebrates or the hemolymph of invertebrates.
Blood plasma is the fluid portion of blood without the cells. It can hold only as much O2 or CO2 as can be dissolved in solution. How much O2 goes into solution at a given partial pressure is a measure of its solubility. Because O2 is about 30 times less soluble than CO2, only about 0.2 ml of O2 can be carried in 100 ml of blood. In both vertebrates and invertebrates, hemoglobin evolved as a specialized iron-containing, or heme, molecule for O2 transport. By binding O2 and removing it from solution, hemoglobin increases the amount of O2 in the blood a hundredfold. Because of its greater solubility, CO2 is transported in solution within the blood. Rather than remaining dissolved in solution as a gas, most of the CO2 (about 95%) is converted to carbonic acid, which dissociates to form bicarbonate ions (HCO3−) and protons (Chapter 6).
Whereas some invertebrate hemoglobins exist in solution in the hemolymph and others exist within cells carried by the hemolymph, all vertebrate hemoglobins are produced by and reside in red blood cells. Hemoglobin gives the cells and the blood their red appearance.
39.3.1 Hemoglobin is an ancient molecule with diverse roles related to oxygen binding and transport.
Hemoglobin is an ancient molecule that has been found in organisms representing all kingdoms of life. In bacteria, archaeons, and fungi, similar forms of hemoglobin evolved as a terminal electron acceptor in cellular respiration and may have also served a role in scavenging O2 to avoid damage due to oxidative stress.
39-11
Plant hemoglobins are known that bind and transport O2 within the cell. In soybeans, hemoglobin binds O2 in root nodule cells to keep concentrations low enough to avoid inhibiting nitrogenase, the enzyme used by symbiotic rhizobial bacteria in the root nodules for nitrogen fixation (Chapter 29).
Invertebrate hemoglobins found in roundworms, annelid worms, and arthropods bind O2 in the circulatory fluid, as discussed above. They share considerable gene and amino acid sequence similarity with vertebrate hemoglobins, indicating that a common ancestor to invertebrate and vertebrate animals evolved a shared form of hemoglobin more than 670 million years ago.
39.3.2 Hemoglobin reversibly binds oxygen.
Hemoglobin exists in large concentrations within red blood cells. It is a globular protein that consists of four polypeptide units (Fig. 39.11). Each of these four units surrounds a heme group that contains iron, which reversibly binds one O2 molecule. After O2 diffuses into the blood, it diffuses into the red blood cells and binds to the heme groups in hemoglobin. Hemoglobin’s binding of O2 removes O2 from solution, keeping the pO2 of the red blood cell below that of the blood plasma so that O2 continues to diffuse into the cell. The removal of O2 from the plasma, in turn, keeps the pO2 of the plasma below that of the lung alveolus, so O2 continues to diffuse from the lungs into the blood.
FIG. 39.11: What is the molecular structure of hemoglobin and myoglobin?
BACKGROUND In the 1950s, the Austrian Max Perutz worked with John Kendrew in the Cavendish Laboratory of the University of Cambridge to determine the molecular structure of globular proteins. Perutz and Kendrew were interested in understanding how the structures of hemoglobin and myoglobin enabled binding and transport of O2.
EXPERIMENT Perutz and Kendrew developed and applied the new technique of X-ray crystallography to determine the three-dimensional molecular structures of hemoglobin and myoglobin.
RESULTS Perutz and Kendrew showed that adult hemoglobin consists of four subunits, two α (alpha) and two β (beta) subunits. Each subunit contains a heme group that contains iron, which is the site of O2 binding. By contrast, myoglobin consists of only a single subunit with one heme group. These differences in molecular structure underlie the O2-binding and dissociation properties of the two O2 transport proteins.
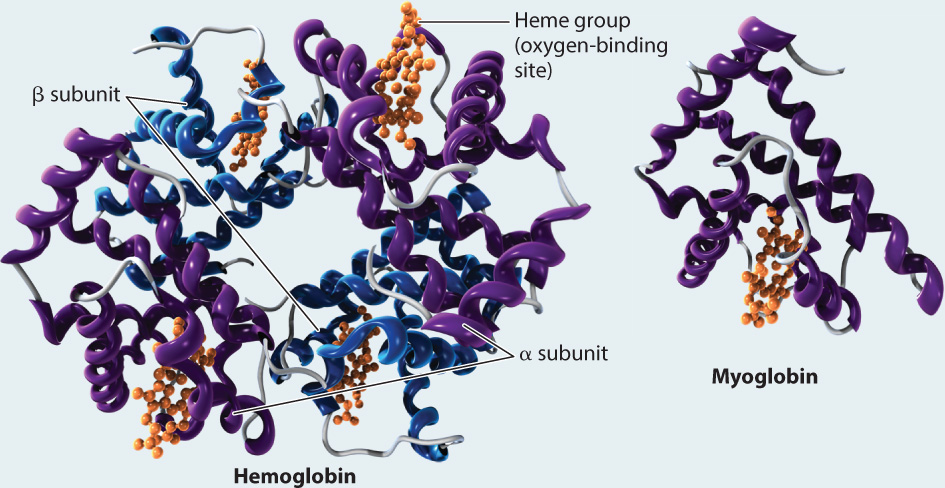
FOLLOW-UP WORK Work by Perutz in the 1960s showed how the molecular structure of hemoglobin changes when it binds O2 versus when it is in an unbound state. Perutz also supervised Francis Crick as a doctoral student, who later worked with James Watson to resolve the structure of DNA. Perutz and Kendrew received the 1962 Nobel Prize in Chemistry for their work.
SOURCES Kendrew J. C., M. F. Perutz. 1948. “A Comparative X-Ray Study of Foetal and Adult Sheep Haemoglobins.” Proceedings of the Royal Society, London, Series A 194:375–98; Perutz, M. F. 1964. “The Hemoglobin Molecule.” Scientific American 211:64–76; Perutz, M. F. 1978. “Hemoglobin Structure and Respiratory Ttransport.” Scientific American 239:92–125.
When more O2 is present in blood plasma, we expect more O2 to become bound to hemoglobin, and that is what happens. But there is an interesting wrinkle that enables hemoglobin to readily bind O2 leaving the lung. If we plot blood pO2 against the percentage of O2 bound to hemoglobin (the relation defined as “hemoglobin saturation”), we get the curve shown in Fig. 39.12a. As blood pO2 increases, hemoglobin saturation rises slowly at first, then more steeply, and then more slowly again until it levels out. At 100% saturation, all hemoglobin molecules bind four O2 molecules. The curve is called the hemoglobin’s oxygen dissociation curve, and it has a sigmoidal shape—that is, a shape like an “S.”
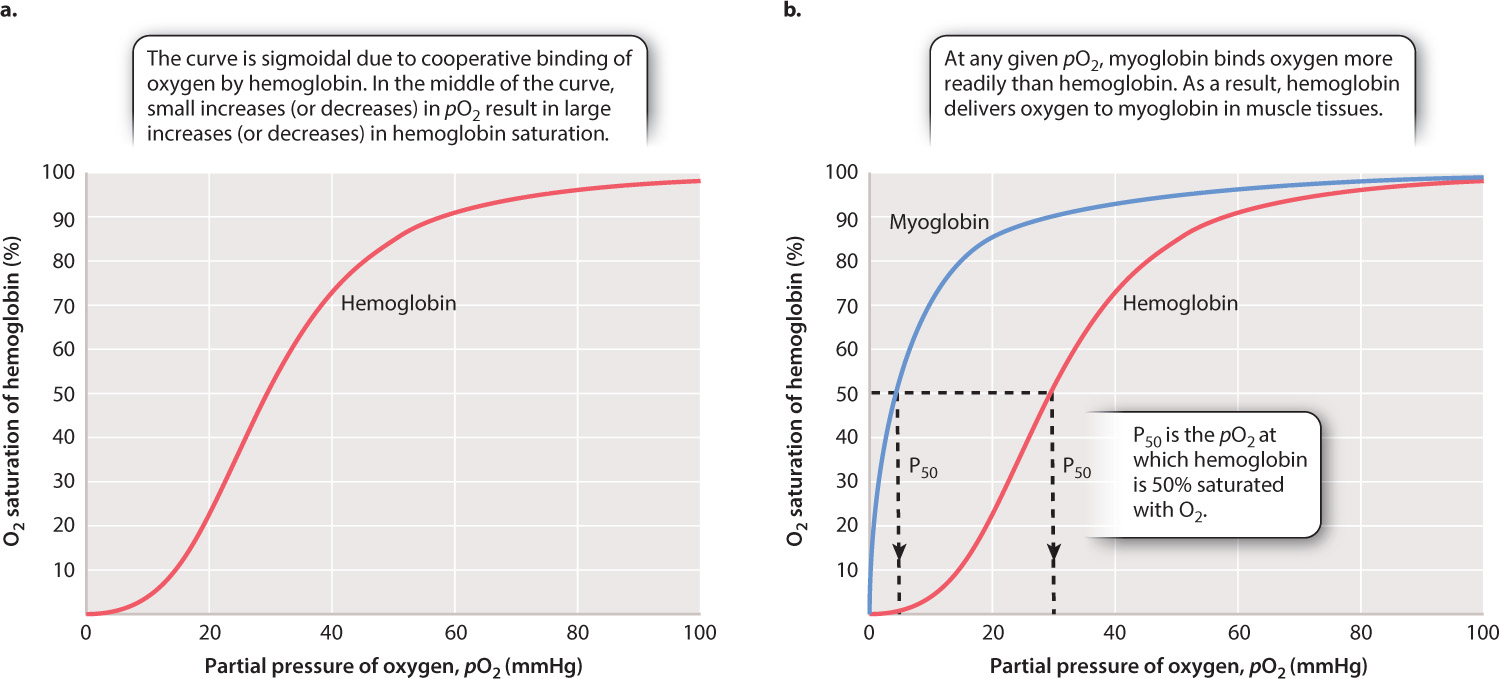
The shape of this curve can be explained as a result of changes in the ability of hemoglobin to bind O2 (a property called its binding affinity) at different O2 partial pressures. At 25% saturation, each hemoglobin molecule binds on average one O2 molecule. As pO2 rises, hemoglobin binds O2 with increasing binding affinity. This increase in affinity results from the interaction of adjacent heme groups in each hemoglobin molecule. After one heme group binds the first O2 molecule, hemoglobin undergoes a conformational change that increases the binding affinity of the remaining heme groups for additional O2. The increase in binding affinity with additional binding of O2 is called cooperative binding, and it gives the O2 dissociation curve for hemoglobin its sigmoidal shape.
39-12
The increasing affinity of hemoglobin for O2 with increasing O2 concentration has important physiological consequences. In the middle part of the O2 dissociation curve, small increases in O2 concentration lead to large increases in hemoglobin saturation. Therefore, under normal circumstances, hemoglobin in the blood leaving the lung is fully saturated with O2 —each hemoglobin molecule is bound to four O2 molecules.
The shape of the O2 dissociation curve also helps to explain how O2 is delivered to respiring cells. When the hemoglobin in red blood cells reaches tissues needing O2 to supply their mitochondria, the O2 is released from the hemoglobin. As active cells consume O2, they reduce the local pO2 of the cell and surrounding tissues to 40 mmHg or less. At these lower pO2 values, the hemoglobin’s O2 dissociation curve has a steep slope (Fig. 39.12a). The steepness of the slope indicates that for a relatively small decrease in pO2, large amounts of O2 can be released from hemoglobin to diffuse into the cell.
39.3.3 Myoglobin stores oxygen, enhancing delivery to muscle mitochondria.
Myoglobin is a specialized O2 carrier within the cells of vertebrate muscles. In contrast to hemoglobin, myoglobin is a monomer that contains only a single heme group. Because there are no interacting subunits, the O2 dissociation curve for myoglobin has a different shape from that of hemoglobin (Fig. 39.12b). In fact, over the physiological range of pO2, myoglobin has a greater affinity for O2 than hemoglobin does and binds O2 more tightly. As a result, hemoglobin releases O2 to exercising muscles. Once the exercising muscles consume the available O2, the intracellular pO2 drops, and the myoglobin releases its bound O2 to the muscle cell’s mitochondria.
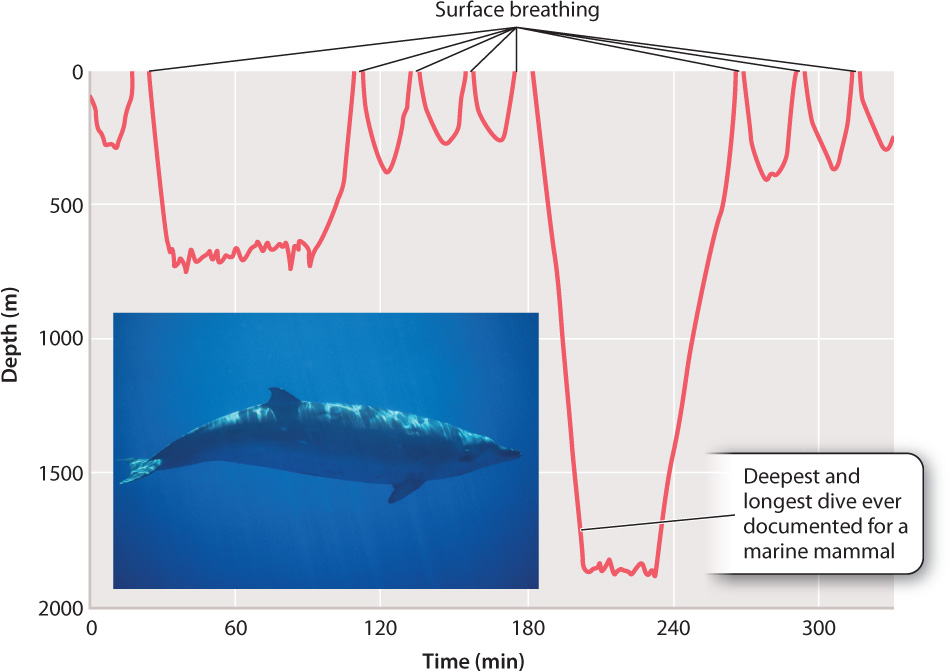
Red muscle cells that depend mainly on aerobic respiration to produce ATP (Chapter 37) store large amounts of myoglobin. The myoglobin in these cells can release O2 quickly at the onset of activity, before respiratory and circulatory systems have had time to increase the supply of O2. Diving marine mammals, such as whales and seals, carry large amounts of myoglobin within their muscles. The myoglobin loads up with O2 when the animals breathe at the surface before a dive. The O2 bound to the myoglobin is then used to supply ATP during the dive, when the animal cannot breathe. These marine mammals can stay under water for 30 minutes or longer and dive to considerable depths (Fig. 39.13).
39.3.4 Many factors affect hemoglobin-oxygen binding.
Obtaining enough O2 is a special challenge in some circumstances. How does a mammalian fetus in its mother’s uterus take up O2 from its mother’s blood? How do animals that live at high altitude obtain O2 under conditions of low pO2? In each case, the species adapts by evolving forms of hemoglobin with higher binding affinity.
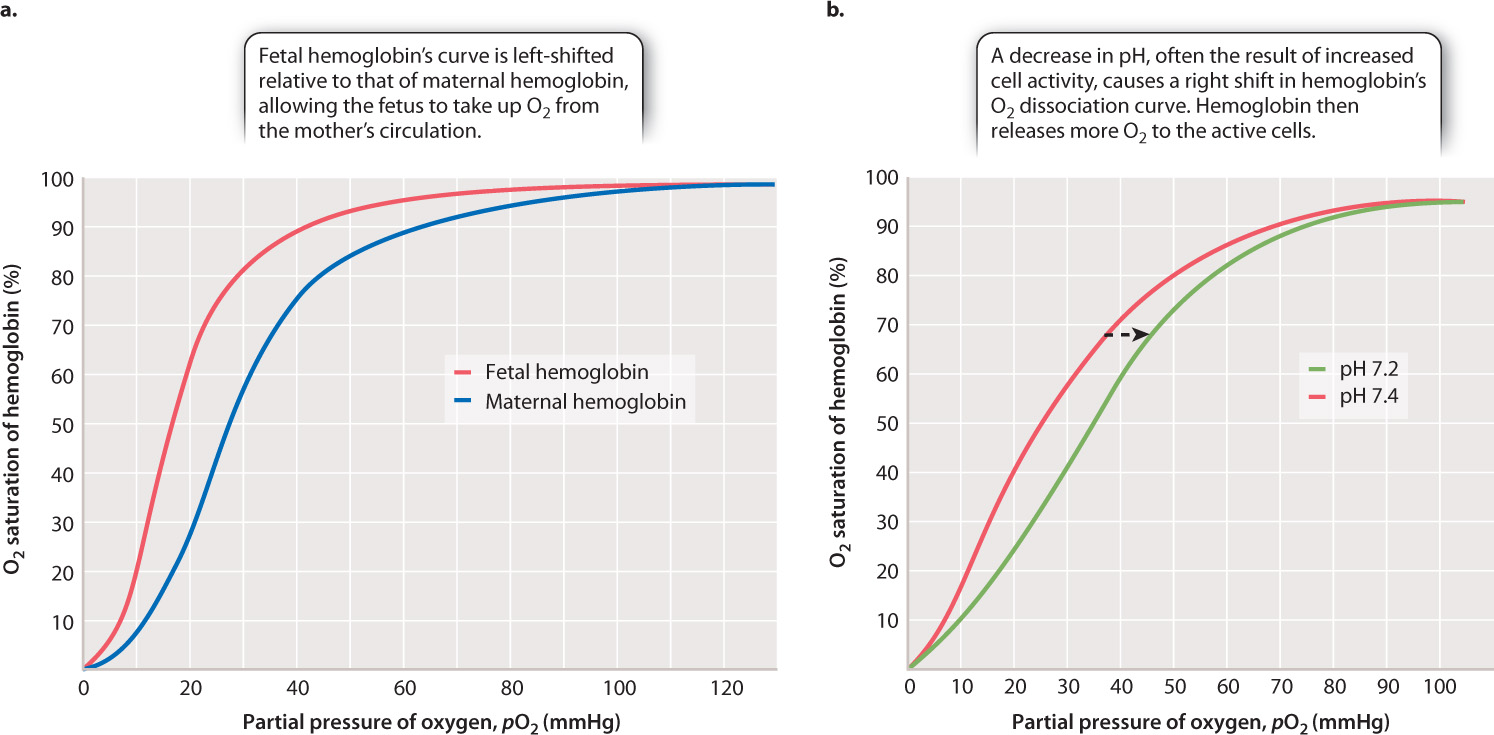
In mammals, the mother’s respiratory and cardiovascular systems must provide the fetus with O2 and remove CO2, as well as supply nutrients and remove waste. Maternal and fetal blood do not mix. Rather, they exchange gases across the placenta. Yet the O2 concentration gradient at this exchange point does not favor the movement of O2 from the mother’s blood to the fetal blood. Fetal mammals solve the problem of extracting O2 from their mother’s hemoglobin by producing a form of hemoglobin before birth that has a higher affinity for O2 than their mother’s hemoglobin. The O2 dissociation curve for fetal hemoglobin is shifted to the left of the curve for maternal hemoglobin (Fig. 39.14a), allowing the fetal hemoglobin to extract O2 from its mother’s circulation. At birth, when the newborn begins to breathe, its red blood cells rapidly shift to producing the adult form of hemoglobin, maintaining this form throughout life.
Many animals live at high altitude. For example, birds have been observed flying over the highest peaks (taller than 9000 m) despite the fact that air at this altitude has less than one-third the O2 content of air at sea level. Similarly, llamas inhabit the Andes at altitudes up to 5000 m, where at a pO2 of approximately 80 mmHg the air has about one-half the O2 content of air at sea level. Consequently, the lung pO2 of a llama is only about 50 mmHg. How do highflying birds and llamas obtain enough O2? Their hemoglobin has a higher affinity for O2 and binds O2 more readily than that of mammals living at sea level. Consequently, their O2 dissociation curve is shifted to the left, favoring the binding of more O2 at a given pO2.
39-13
Tissue and blood pH also has an important physiological effect on the O2 affinity of hemoglobin. When a decrease in pH (that is, an increase in H+ concentration) occurs during exercise, the affinity of hemoglobin for O2 decreases, resulting in a rightward shift in the O2 dissociation curve (Fig. 39.14b). This phenomenon is called the Bohr effect after Christian Bohr, the Danish physiologist who first described it in 1904. Decreases in pH occur when CO2 is released from metabolizing cells, or when an inadequate supply of O2 leads to the production of lactic acid (Chapter 7). Because hemoglobin’s affinity for O2 is reduced, more O2 is released and supplied to the cells for aerobic ATP synthesis.
Carbon dioxide also reacts with the amine (NH2) groups of hemoglobin, reducing hemoglobin’s affinity for O2. Thus, when released from respiring tissues, CO2 promotes increased O2 delivery both through its direct effect on hemoglobin and its contribution to a decrease in blood pH by the Bohr effect. While the production of CO2 promotes O2 release at the tissues, its elimination at the lung increases hemoglobin’s affinity for O2, enhancing O2 uptake.