41.1 WATER AND ELECTROLYTE BALANCE
A healthy person can live several weeks without food but cannot survive much more than a few days without water. A loss of more than 10% of body water threatens survival. Homeostasis of water and electrolyte concentration is critical to normal physiological function, not just in humans, but in all organisms.
41-2
41.1.1 Osmosis governs the movement of water across cell membranes.
Water, like all molecules, moves by diffusion, or the random motion of molecules. When there is a difference in water concentration between two regions, there is a net movement of water molecules from regions of higher water concentration to regions of lower water concentration, a process called osmosis (Chapter 5). Water often contains other dissolved molecules, such as electrolytes, amino acids, and sugars, which are collectively called solutes. Because the concentration of a solute is typically known and the concentration of water is not, it is sometimes easier to think about water moving from regions of lower solute concentration (higher water concentration) to higher solute concentration (lower water concentration). Whether in terms of water concentration or solute concentration, the direction of net water movement is the same.
In organisms, the ability to control osmosis, and therefore to regulate levels of water and electrolyte in cells, depends on the properties of their plasma membranes and embedded proteins. When a barrier allows water or solutes to diffuse freely, it is said to be permeable. When it blocks the diffusion of water or solutes entirely, the membrane is considered impermeable. When it allows the movement of some molecules but not others, it is semipermeable, or selectively permeable. The membranes of cells are selectively permeable because of the combination of the lipid bilayer and transmembrane proteins, as we saw in Chapter 5.
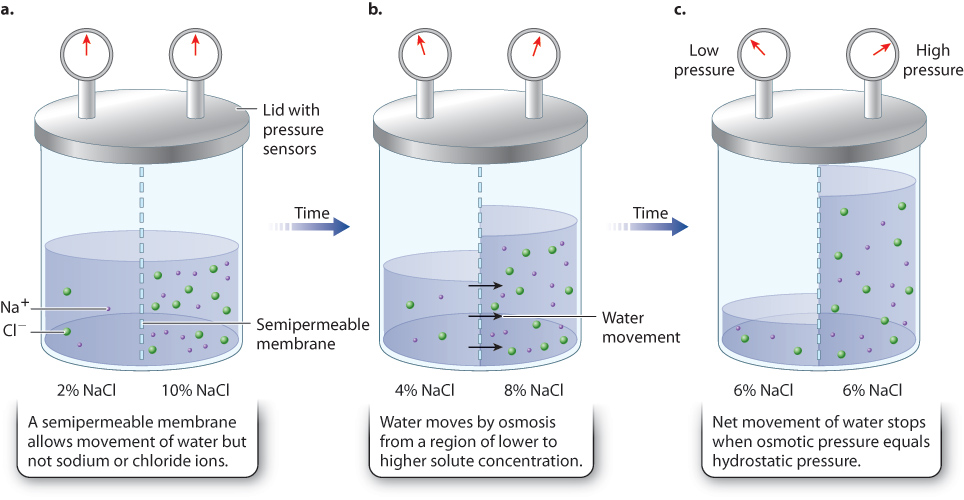
Osmosis governs the movement of water across semipermeable membranes (Fig. 41.1). Plasma membranes and sheets of cells that are semipermeable allow water and smaller solutes to diffuse freely but prevent larger solutes, like proteins, from passing through them. Water is able to move directly through lipid bilayers by diffusion or through channels called aquaporins by facilitated diffusion (Chapter 5).
Consider then a situation in which there is a difference in solute concentration on the two sides of a semipermeable membrane, and the membrane is impermeable to the solute (Fig. 41.1a). Water moves by osmosis from regions of higher water concentration to regions of lower water concentration, or, from regions of lower solute concentration to regions of higher solute concentration (Fig. 41.1b).
Question Quick Check 1
yt7esYdQiosOhNAOrLdAvxr0pjoMKdCu7Qj8DcNdwbov5J0/0u+T5i625Zz9J+Hmp7WJI9tUqMua/gpdswr5V0BxARLh2jxBPhG/pS8+IH7MOlhbF/AmGmCPi0hrPekKicqmsErAVsob4tVGL6Oa1FEul6qwSctqNK7EyIwXG/aPXsWaSsvEzOK+HgjOLCcuobf7R53I0BDbAelqcVizHOG1gsbweGe8kzGcvTHDsozPSCDzw9TVZUl/I3gbAcOMPtKsp0zGPy3/n7SIP7fJPj7zRC1uMUWDEq62TLysrxoNOqm+TscWwbBHyTItwD5ot4xoLfOS1oI=The difference in concentration of solutes on the two sides of the membrane creates osmotic pressure that drives the movement of water. Osmotic pressure is the tendency of water to move from one solution into another by osmosis. The higher the solute concentration, the higher the osmotic pressure of that solution (that is, there is a greater tendency for water to move into that solution). Water diffuses into a region with a higher solute concentration (higher osmotic pressure) until it exerts a hydrostatic pressure (the pressure exerted by water itself due to gravity, not differences in solute concentration) that balances the osmotic pressure. When osmotic pressure equals hydrostatic pressure, the net movement of water stops and equilibrium is reached (Fig. 41.1c). At equilibrium, water molecules still move across the membrane in both directions by diffusion, but there is no net movement of water molecules.
41-3
Whether osmotic pressure drives water into or out of a cell depends on the solute concentration of the inside of the cell relative to the outside. Similarly, whether osmotic pressure drives movements of water into or out of an organism depends on solute concentration inside an animal relative to its environment. Note that osmosis is driven by differences in total solute concentration between the two sides of a cell membrane. That is, if a particular electrolyte such as potassium has a high concentration inside the cell and low concentration outside a cell, but another electrolyte, such as sodium, has exactly the opposite distribution, there will be no net water movement because the total concentrations of the solutes on the two sides of the membrane are the same. Also note that water moves across semipermeable membranes in response to concentration differences of any solute. That is, the solute can be an electrolyte, such as sodium or potassium, or any other dissolved molecule, such as glucose or an amino acid or even waste, as long as the membrane is impermeable to it.
Question Quick Check 2
cYX1YNqFhPACAS18e9XuBDcA4YA5xVOh8fDqPutzQLh4tUuEtoYKcWfVgEEaAggt1/XWlp5PCZB8yT2B3ieG0V3UMatZ5C8SAPprhA1upu+ZC0N9XKdvtSFlphG0qA4rS62V+EEYJw/t7WlC7b1bfa14vkzU4KpLZl++bs+rWaY4y2QjXIiiVeU2uDon9GRlwdF0aCI0rVmMrNqKrat1N6RrY9VX9b6TUpUMlYeWMoYWMxEHCjvx1jOIfSWpilFkBysMruLrFDND7gmnGyQwUew8s4W02KFboGa0DOmiSDNdIkQcH572OENs1GEFIuUgUoCCXv6oRJXizR1KU2sBG1/pibvv6enBBWd6rcbw4oT22xw4LrlvgJXVJucbSBtxByFskkpDvz6MeI53j75e0tuIodVpNASF41.1.2 Osmoregulation is the control of osmotic pressure inside cells and organisms.
Animal life on Earth exists in a wide range of environments by adopting different means of osmoregulation, or the regulation of water and solute levels to control osmotic pressure. Osmoregulation, like energy balance (Chapter 40), is a form of homeostasis. High osmotic pressures inside a cell can damage the cell, sometimes even causing it to burst and thus disrupt some of the animal’s functions. Although cells and tissues can tolerate dehydration, often as high as 50% or more, excessive dehydration impairs a cell’s metabolic function, as many chemical reactions, such as the hydrolysis reactions that build proteins and nucleic acids from individual subunits, depend on the presence of water.
How do cells control their internal osmotic pressure? To regulate water and solute levels, and hence the osmotic pressure inside cells, the cell controls the solute concentration of the inside of the cell relative to the outside of the cell because water follows solutes across semipermeable cell membranes. At the level of the cell, then, osmoregulation is achieved by the movement of solutes, particularly electrolytes.
At the level of the organism, osmoregulation is achieved by balancing input and output of water and electrolytes. The water that animals need is gained and lost in different ways. Fig. 41.2 shows sites of water gain and loss in a saltwater fish, which vary depending on the organism. In general, animals gain water by drinking water that is less concentrated in solutes (hypotonic) than their body fluids. Humans and many other vertebrates, both freshwater and terrestrial, gain most of their water this way. Water is also acquired through the food that animals eat. Additionally, animals produce water as a product of cellular respiration when two protons (H+) combine with oxygen (O2) to form water (Chapter 7). This is the primary source of water for some animals adapted to deserts and oceans, where drinkable water is not readily available. Animals lose water in their urine and feces. Terrestrial animals lose water through evaporation from their lungs, and, in the case of humans, by sweating. Freshwater animals gain water through their gills, whereas most marine animals lose water through their gills.
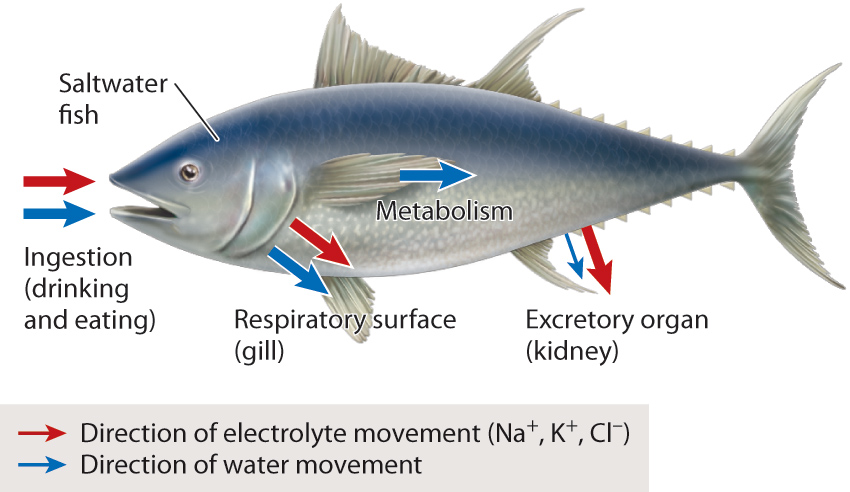
Similarly, electrolytes are taken in and lost in different ways. Animals gain electrolytes in the food they eat. If the water they drink has a higher solute concentration than their body fluids, animals can gain electrolytes by drinking. Marine aquatic animals gain electrolytes as hypertonic water moves across their gills and body surface. In contrast, freshwater aquatic animals lose electrolytes through diffusion across their gills and skin into the hypotonic watery environment. Humans lose electrolytes as well as water when they sweat. Drinking beverages high in electrolytes is helpful before demanding physical activities—including sports—because these activities can result in substantial water and electrolyte loss through sweating. Electrolytes are also lost by specialized glands (discussed below) and in urine and feces.
The ability of many organisms to regulate their water and electrolyte levels has enabled them to maintain homeostasis and a stable body chemistry in the face of changing environmental conditions, and to live in diverse environments. Most forms of life originated in salty marine environments, but many groups of organisms subsequently moved onto land and into freshwater lakes and rivers that present challenges for water and electrolyte balance. Living in freshwater environments requires animals to avoid excess water uptake into their tissues and control the loss of electrolytes from their body. Living on land requires animals to minimize their water loss in order to avoid desiccation, or drying out. Only insects and terrestrial vertebrates have achieved the ability to restrict water loss.
41-4
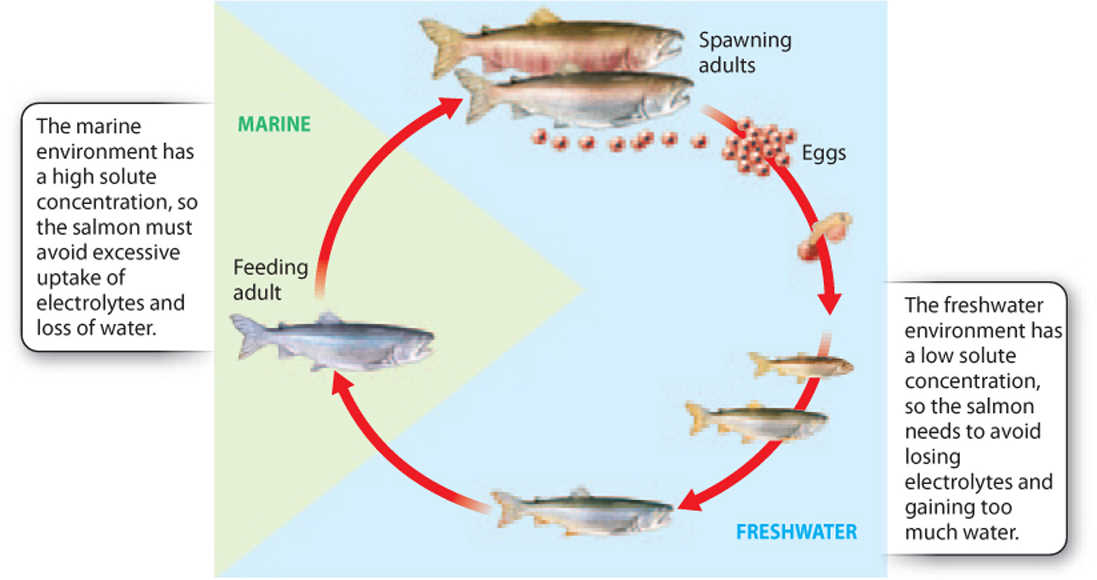
An extreme example of the ability of an animal to regulate water and electrolyte levels is provided by the life cycle of salmon. Mature salmon swim in open ocean saltwater environments but return upriver to spawn in freshwater environments (Fig. 41.3). As they do, they swim from high-salt ocean water through water of intermediate saltiness into freshwater rivers and streams that have little salt. After spawning, the young feed and grow in freshwater over a period of up to 3 years, eventually returning to the open ocean, where they live until they become reproductively mature and return to their home river system to spawn again.
These changes in aquatic habitat demand that the salmon make dramatic adjustments in the way they handle water and solutes. When in the ocean, the salmon must avoid excessive uptake of electrolytes and loss of water. However, when mature salmon migrate upriver to spawn and as young salmon continue to grow in freshwater streams and rivers, the challenge is reversed: They must avoid losing critical electrolytes and gaining too much water across their gills. In salmon, radical changes in osmoregulation are required over the course of an animal’s lifetime to enable reproduction in freshwater streams and a return to the sea for further growth and maturation. Next, we explore how gills and kidneys meet these challenges.
41.1.3 Osmoconformers match their internal solute concentration to that of the environment.
Animals regulate their internal osmotic pressure in different ways. Some animals match their internal osmotic pressure to that of their external environment. These animals are osmoconformers. They keep their internal fluids at the same osmotic pressure as the surrounding environment, which reduces the movement of water and solutes into or out of their bodies. Because the solute concentrations inside and outside osmoconformers are similar, osmoconformers don’t have to spend a lot of energy regulating osmotic pressure. However, they have to adapt to the solute concentration of their external environment, which is typically seawater. Osmoconformers tend to live in environments with stable solute concentrations, which provide an effective way of maintaining stable internal solute concentrations.
Although osmoconformers generally match the overall concentration of their tissues with their external environment, they often expend energy to regulate the concentrations of particular ions such as sodium, potassium, and chloride (Cl–), and other solutes such as amino acids and glucose that are important to their function. For example, whereas the intracellular space of nearly all multicellular animals has a relatively high concentration of potassium ions and low concentration of sodium ions, the proportion in the extracellular space of most animals is just the opposite—low in potassium but high in sodium. This means that these cells are actively pumping sodium out and potassium in (Chapter 5).
Most marine invertebrates are osmoconformers, matching their total intracellular solute concentration to the solute concentration of seawater. These organisms maintain high concentrations of sodium and chloride in their cells to achieve an overall solute concentration close to that of seawater. Consequently, they have few specializations for osmoregulation beyond the need to regulate specific internal ion concentrations relative to their environment. Many marine vertebrates are also osmoconformers. Some, like hagfish and lampreys, match their total internal solute concentration to that of seawater but maintain high internal concentrations of particular electrolytes, just as marine invertebrates do.
Other marine vertebrates that are osmoconformers, like sharks, rays and coelocanths, match seawater’s solute concentration by maintaining a high internal concentration of a compound called urea. Urea is a waste product of protein metabolism that many animals excrete, so it does not build up to high concentrations (section 41.2). By retaining this solute, these marine vertebrates are able to achieve osmotic equilibrium with the surrounding seawater. Even though marine osmoconformers seek to match the osmotic concentration of their environment, these animals also regulate specific ions for particular physiological functions within their body, as discussed in more detail below.
41-5
41.1.4 Osmoregulators have internal solute concentrations that differ from that of their environment.
Many animals maintain internal solute concentrations that differ from that of their environment. These animals are osmoregulators. Osmoregulators expend considerable energy pumping ions across cell membranes in order to regulate the movement of water into or out of their bodies. Some freshwater and marine fishes that are osmoregulators are estimated to expend 50% of their resting metabolic energy on osmoregulation. However, the ability to regulate osmotic pressure allows osmoregulators to live in diverse environments—saltwater, freshwater, and land.
The external surface (such as the skin) of most osmoregulators prevents water and solutes from passing freely between the environment and the inside of the animal. Although the animal’s skin maintains different total concentrations of solutes between the outside and inside of the animal, most of the internal cells do not maintain differences in total solute concentrations on the two sides of the plasma membrane. As a result, in general, there are usually no lasting differences in osmotic pressure between the inside and outside of internal cells of osmoregulators.
Teleosts, or bony fish (the largest group of marine vertebrates), as well as all freshwater and terrestrial animals, are osmoregulators. These animals have evolved a variety of specializations for water and electrolyte balance and waste excretion. Whereas marine teleosts maintain concentrations of electrolytes much lower than the surrounding seawater, freshwater fishes and amphibians maintain concentrations that are higher than those of their aquatic environment.
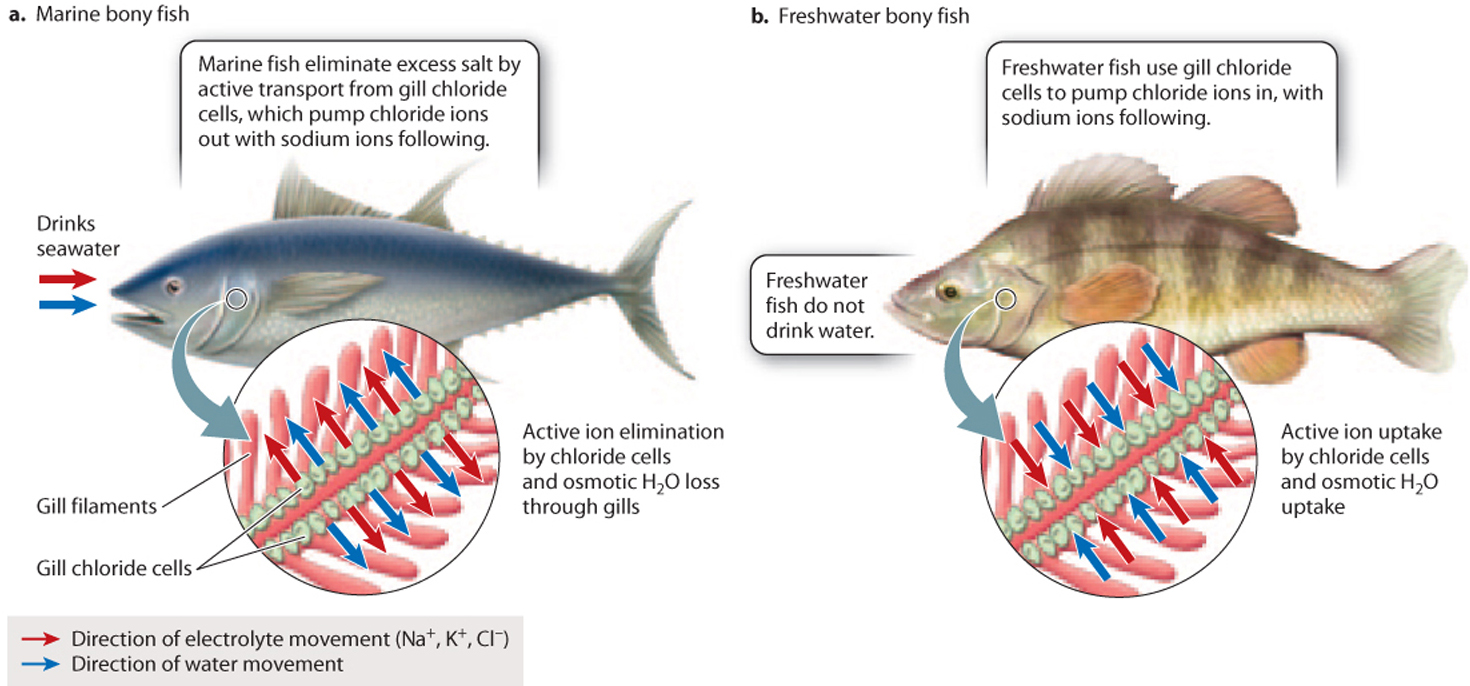
Aquatic animals use their gills to breathe as well as to regulate electrolyte levels. The thin gill filaments, with their extensive surface area, are well adapted for gas exchange and also facilitate electrolyte transport (Fig. 41.4). In marine bony fishes, specialized cells in the gills called chloride cells counter the ingestion and diffusion of excess electrolytes into the animal by pumping chloride ions into the surrounding seawater. By pumping negatively charged chloride ions out of the body, chloride cells create an electrical gradient that is balanced by positively charged sodium ions moving out of the body as well (Fig. 41.4a). In contrast, freshwater fishes have gill chloride cells with reversed polarity. Their chloride cells pump chloride ions (with sodium ions following) in the reverse direction, moving the chloride ions into the body to counter their ongoing loss from the gills into the surrounding freshwater (Fig. 41.4b). In both cases, the gills maintain a substantial difference in solute concentration between the fish’s internal and external environment.
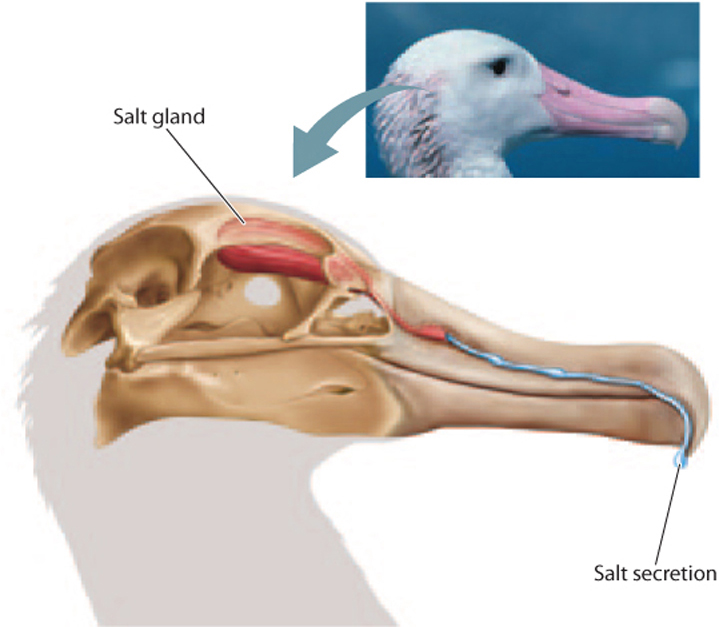
Sharks and rays excrete excess salt by a rectal gland, which eliminates salt with their digestive wastes. Similarly, many marine birds have evolved specialized nasal salt glands to rid themselves of the high salt content of their diet (Fig. 41.5). These salt glands work by actively pumping ions from the blood into cells that make up the gland, followed by excretion of salt from the body through the gland and the nasal cavity. Their salt glands allow these animals to gain net water intake by drinking seawater. In contrast, humans and many other non-marine-adapted animals are incapable of deriving useful net water gain by drinking seawater because they cannot eliminate salt in high enough concentration. The high concentration of magnesium sulfate in seawater results in diarrhea if too much is consumed, exacerbating the net loss of water in the feces.
41-6
Some organisms that encounter a range of environments use different means at different times. For example, the brine shrimp Artemia usually acts as an osmoconformer but can act as an osmoregulator in extreme environments. Artemia lives in salt lakes such as the Great Salt Lake of Utah, where it usually matches its internal solute concentration to that of the outside environment. However, it sometimes encounters exceptionally high salt concentrations. It can survive in this high-salt environment by excreting excess salt ions from its gills to maintain an internal concentration that is hypotonic relative to its environment. Alternatively, in low saltwater environments (but not in freshwater, which they cannot tolerate), Artemia reverse their gill ion exchange and maintain a hypertonic internal body fluid concentration relative to the surrounding water.
In addition to respiratory surfaces such as gills and specialized salt glands, the other major regulator of water and electrolyte levels is the kidney, which we discuss in the next two sections.
41.1.5 Can the loss of water and electrolytes in exercise be exploited as a strategy to hunt prey?
Case 7 Predator–Prey: A Game of Life and Death
As we have seen, exercise can lead to loss of water and electrolytes, a response that is exploited by African bushmen as they hunt large prey. African bushmen are hunter-gatherers; their tribes live in hot, dry grasslands. Equipped only with wood spears, bows, and poisoned arrows, they have historically hunted kudus, antelope, buffalo, and other large grazing herbivores (Fig. 41.6). Bushmen hunters pursue and track down their prey, exploiting effects of the physiological stress that occurs when their prey run during the heat of the day.

Before hunting, bushmen drink as much water as possible, hydrating their bodies. They select a large prey animal, which they chase from a shaded refuge into the open sun when temperatures exceed the animal’s core body temperature. The bushmen can deal with the high temperatures, cooling their bodies through sweating, but because they hydrated thoroughly before the hunt, their sweating does not dehydrate them. Not so for the prey. The bushmen hunters track and chase their prey until the prey becomes dehydrated and hyperthermic, thus rendered unable to escape.
41-7
Even if the bushmen hunters strike the animal successfully with a poisoned arrow, they still must track it for long distances and over long periods of time. Hunts typically last several hours, but sometimes as much as 1 or 2 days, and cover distances of 25 km (about 16 miles) or more. The loss of water in intensive heat disrupts the animal’s water and electrolyte balance and thus contributes to its inability to move and defend itself.