43.3 ADAPTIVE IMMUNITY: T CELLS AND CELL-MEDIATED IMMUNITY
Antibodies are remarkable for their diversity and specificity. They provide protection against many kinds of bacteria, especially those encased in a polysaccharide capsule, such as the bacteria that cause pneumonia and meningitis. The B cells that generate them exhibit immunological memory, as we saw by contrasting the primary and secondary immune responses. However, B cells and their antibodies have limitations. For example, B cells on their own can make antibodies only against some antigens; for other antigens, they require the assistance of other cells. Furthermore, they are sometimes ineffective against pathogens that take up residence inside a cell, as do the bacteria that cause tuberculosis.
To handle these and other kinds of pathogens, there is another part of the adaptive immune system, which depends on the second type of lymphocyte—the T cell. T cells do not secrete antibodies. Instead, they participate in cell-mediated immunity, so named because cells, not antibodies, recognize and act against pathogens. The importance of cell-mediated immunity is most dramatically demonstrated by individuals taking immunosuppressive drugs or infected by HIV. Both of these suppress the activity of T cells. The result can be overwhelming infections and even tumors.
43.3.1 T cells include helper and cytotoxic cells.
T cells, like B cells, originate in the bone marrow. However, unlike B cells, T cells mature in the thymus. A mature T cell is characterized by the presence of a T cell receptor (TCR) on the plasma membrane. The T cell receptor is a protein receptor that recognizes and binds to the antigen. In this way, the T cell receptor is similar to an antibody. However, whereas antibodies may be either on the cell surface of B cells or secreted, TCRs are always found on the cell surface of T cells.
There are two major subpopulations of T cells with different names, functions, and cell-surface markers—helper T cells and cytotoxic T cells (Table 43.2; see Fig. 43.3). Helper T cells do just that—they help other cells of the immune system by secreting cytokines. One of their key roles is to activate B cells to secrete antibodies. Although B cells can work on their own, particularly against encapsulated bacteria, in most cases they require the participation of helper T cells. Helper T cells also activate macrophages, cytotoxic T cells, and other cells of the immune system.
| Comparison of Helper and Cytotoxic T Cells | ||
|---|---|---|
| HELPER T CELL | CYTOTOXIC T CELL | |
| Functions | Activation of macrophages Activation of B cells Activation of cytotoxic T cells Secretion of cytokines |
Cell killing |
| Surface molecule | CD4 | CD8 |
| MHC molecule recognized by the T cell | Class II | Class I |
Cytotoxic T cells, as their name implies, are cytotoxic—they can kill other cells. Like B cells, they are activated by cytokines released from helper T cells. They are particularly effective against altered host cells, such as those infected with a virus or that have become cancerous.
These two types of T cell are distinguished by the presence of different glycoproteins on their surface—CD4 on helper T cells and CD8 on cytotoxic T cells. The ratio of these two classes of T cell can be used to assess immune function. In healthy individuals, the CD4:CD8 ratio is usually about 2. A decreased ratio, indicating the presence of fewer helper T cells relative to cytotoxic T cells, is typical of individuals infected with HIV, the virus that causes AIDS. HIV infects and kills helper T cells, disarming a key player in the immune system.
43-14
43.3.2 T cells have T cell receptors on their surface.
T cells have TCRs on their surface that recognize and bind antigens (Fig. 43.15). In several respects, TCRs are similar to antibodies on the surface of B cells. For example, TCRs recognize antigens with a specific structure. In addition, there is a great diversity of TCRs that differ from one another, but each T cell has just one type of TCR on its surface. Binding of TCR to an antigen triggers the T cell to divide into clones, resulting in a pool of T cells that are each specific for a given antigen. Finally, the diversity of TCRs among different T cells results from genomic rearrangement of V, D, J, and C gene segments.
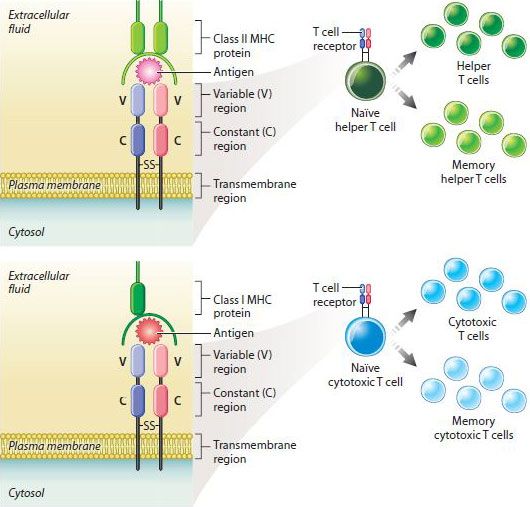
However, TCRs are different from antibodies in important ways. First, they are composed of two, rather than four, polypeptide chains. Second, they are not secreted like antibodies, but are always membrane bound on the T cell surface. Finally, the TCR does not recognize free antigen. It only recognizes antigen in association with proteins of the major histocompatibility complex, or MHC, proteins that appear on the surface of most mammalian cells (Fig. 43.15).
Once activated, T cells divide and form helper and cytotoxic T cells. Some cells of each type are memory cells that provide long-lasting immunity following an initial infection, as in the case of B cells (Fig. 43.15). Also like B cells, T cells can sometimes be activated too strongly. Earlier, we saw how IgE bound to mast cells and basophils can lead to immediate hypersensitivity reactions characteristic of allergies and asthma. The counterpart in T cells is a delayed hypersensitivity reaction, which, as its name suggests, does not begin right away. For example, if you touch poison ivy, your skin will turn red and start itching only after a delay of several hours or days. Delayed hypersensitivity reactions are initiated by helper T cells, which release cytokines that attract macrophages to the site of exposure, which is typically the skin.
43.3.3 T cell activation requires the presence of antigen in association with MHC proteins.
Because of their central role in activating different components of the immune system, T cells themselves require not one signal but two signals to become activated. One of these signals is an antigen, which indicates the presence of foreign cells or particles. The second signal is MHC proteins. T cells interact only with antigens that are bound to molecules of the MHC on the surface of host cells. The requirement for antigen in association with MHC proteins explains why T cells target infected or cancerous host cells. MHCs were first discovered in transplantation biology because it is their presence that leads to acceptance or rejection of transplanted tissues.
43-15
The MHC is a cluster of genes present in all mammals that encode proteins on the surface of cells. The MHC is composed of many genes with a high rate of polymorphism, meaning that there is a lot of variability in the gene sequence (and consequently the protein sequence) among individuals. In humans and mice, the genes are divided into three classes: class I genes are expressed on the surface of all nucleated cells; class II genes are expressed on the surface of macrophages, dendritic cells, and B cells; and class III genes encode several proteins of the complement system and proteins involved in inflammation.
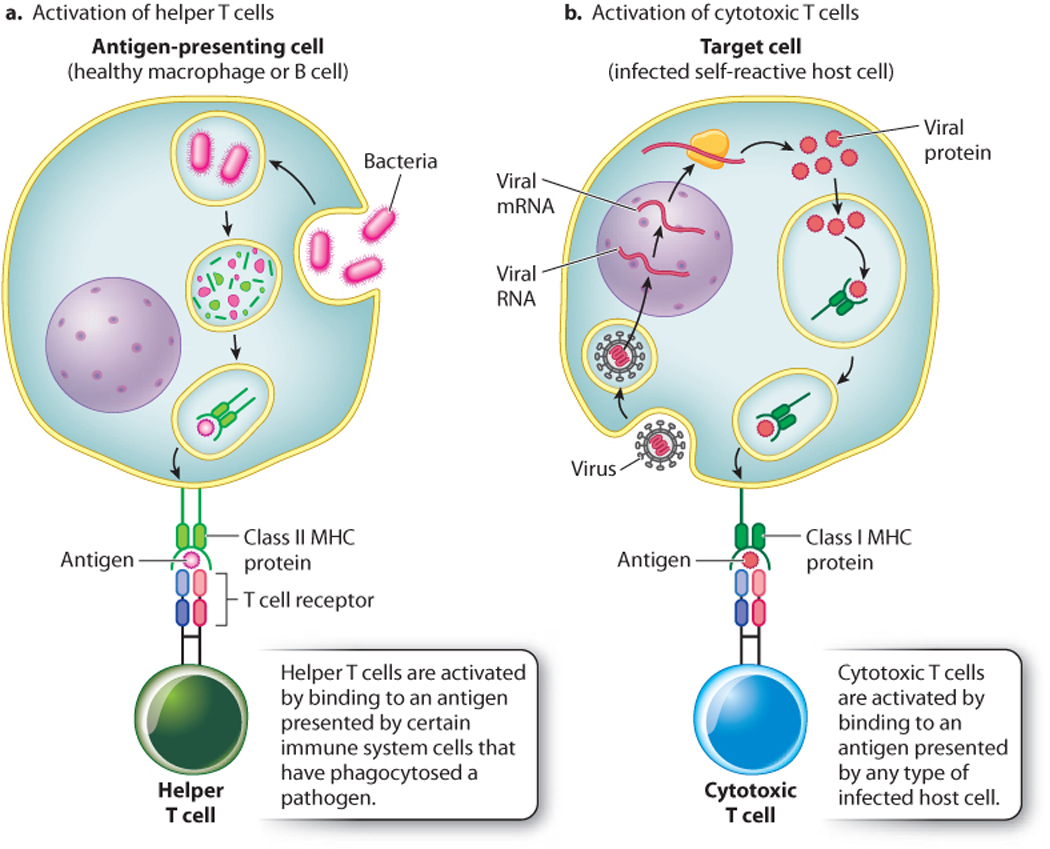
Let’s consider the activation of helper T cells (Fig. 43.16). When an antigen enters the immune system, it may be recognized by an antibody directly or be taken up by antigen-presenting cells. These cells, which include macrophages, dendritic cells, and B cells, take up the antigen and return portions of it to the cell surface bound to MHC class II proteins. Helper T cells recognize processed antigen along with MHC class II molecules by their T cell receptors. As a result of TCR binding to antigen and MHC class II proteins, the helper T cells release cytokines that activate other parts of the immune system, including macrophages, B cells, and cyotoxic T cells.
Cytotoxic T cells also recognize antigen displayed by host cells, but in this case the antigen is presented in association with MHC class I molecules (Fig. 43.16). Because class I molecules are present on virtually all cells, cytotoxic T cells recognize and kill any host cell that becomes abnormal in some way. For example, a virus-infected cell often expresses viral antigens and MHC class I molecules on its surface. Cytotoxic T cells recognize the antigen and MHC class I molecules and kill the cell. Tumor cells express novel antigens along with MHC class I proteins, and may also be eliminated in some cases by cytotoxic T cells.
Question Quick Check 4
MrO7C9pv2z8Mi5I24CWnVlDpDVLB0FdCcLjRo4/HW3jtUu/BZkRvnKrl93gChIKGzOkH0wytBENlvo5+tMueCg==43.3.4 The ability to distinguish between self and non-self is acquired during T cell maturation.
Of the possible T cell receptors that are generated by genomic rearrangement, only some are useful: those that react with the host’s own MHC molecules. In addition, useful T cell receptors must not react with molecules normally present in or on cells of the host. In other words, T cells must not be activated in response to an organism’s own cells. Molecules normally present on a host cell that can bind antibodies are called self antigens.
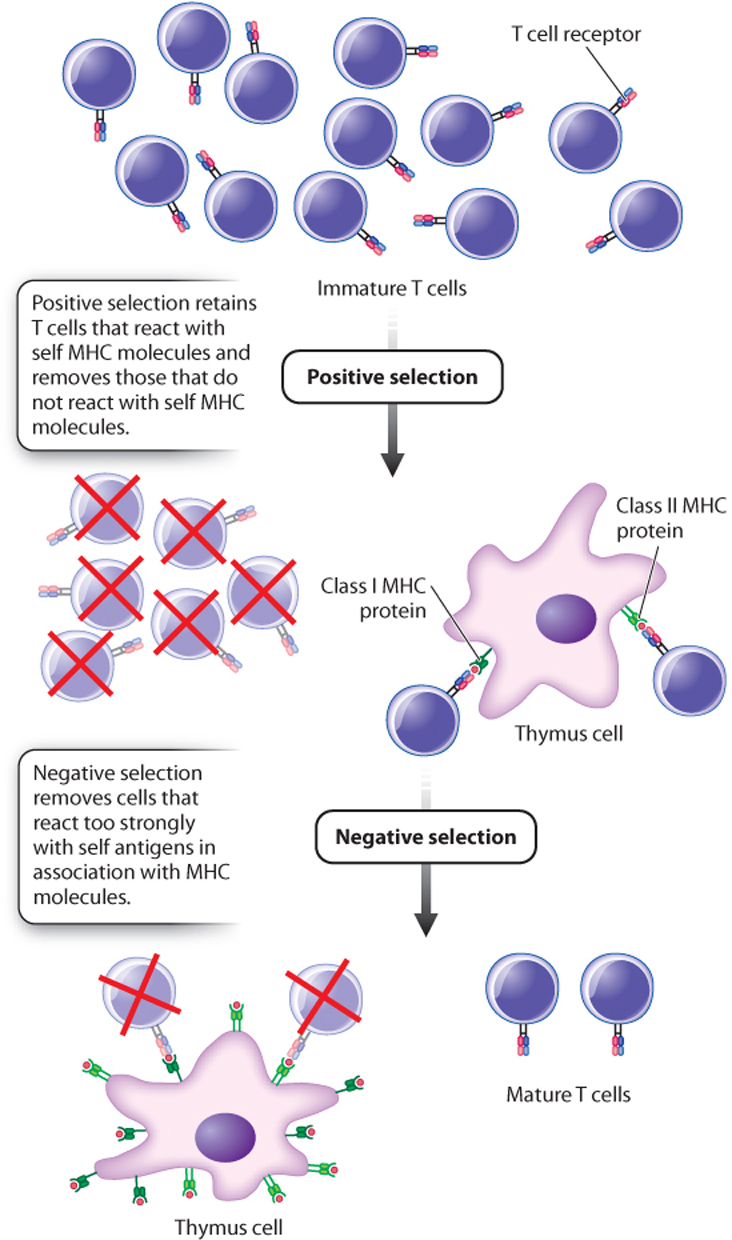
A sorting process is therefore necessary so that only some T cells mature and others are eliminated (Fig. 43.17). As T cells mature in the thymus, they interact with cells of the epithelium of the thymus. Those that recognize self MHC molecules on epithelium cells are positively selected and continue to mature. Those that react too strongly to self antigens in association with MHC are negatively selected and eliminated through cell death. Note that, in spite of their names, both processes involve elimination of some T cells. By far, the majority of T cells are eliminated through a combination of positive and negative selection.
The result of this sorting process is twofold. First, T cells become MHC restricted, so helper T cells interact with foreign antigen plus MHC class II proteins and cytotoxic T cells interact with foreign antigen plus MHC class I proteins. Second, T cells exhibit tolerance—in other words, they do not respond to self antigens, even though the immune system functions normally otherwise. Those antigens present as T cells mature are labeled as self and do not elicit a response; those not present are non-self and, if encountered, do elicit a response.
B cells are not MHC restricted. However, they, too, exhibit tolerance to self antigens because they go through a similar process of negative selection in the bone marrow. During that process, B cells that react strongly to self antigens are eliminated through cell death. Although most self-reactive T and B cells are eliminated, some T and B cells that react to self antigens escape this developmental check and end up in the circulation. As a result, additional mechanisms are in place to eliminate these self-reactive cells, either through the activity of T cells or through cell death.
The ability to distinguish self from non-self is critical. Failure leads to autoimmune diseases, in which tolerance is lost and the immune system becomes active against antigens of the host. Autoimmune diseases can be debilitating as T cells or antibodies attack cells and organs of the host. In rheumatoid arthritis, self-reactive T cells attack the joints, causing inflammation and tissue damage; in type I diabetes, attack is directed against insulin-producing cells of the pancreas; and in multiple sclerosis, the target is the myelin sheath surrounding nerves of the central nervous system. It is not fully understood how these self-reactive T cells evade checks on their development. Perhaps one clue is that some autoimmune diseases are associated with particular MHC alleles.