45.2 GENES AND BEHAVIOR
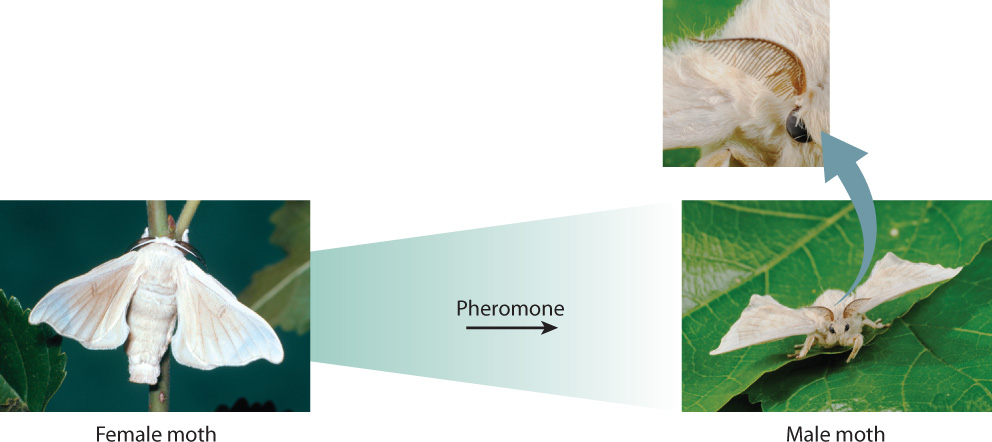
The answers to Tinbergen’s questions rely on an interplay between genes and the environment. The influence of genes is especially clear in innate behaviors, those that are instinctive and carried out regardless of earlier experience. Fig. 45.1 shows that a male silkworm moth of the genus Bombyx flies upwind toward the source of a female-produced pheromone, an airborne chemical signal that communicates with members of the same species, in this case to attract the opposite sex. Pheromones released from a female’s abdominal gland are sensed by small hairs on the male’s antennae. In the presence of pheromones, the antennal sensory hairs fire action potentials (Chapter 35). When about 200 hairs are activated per second, a male flies upwind distances of a kilometer or more, tracking the increasing pheromone concentration until he finds the female. The male does not need to learn this behavior; he performs it spontaneously. His genes encode molecular receptors to which the pheromone binds, and this binding triggers a cascade of events that result in the moth heading up the pheromone’s concentration gradient.
A particular behavior may also depend on an individual’s experience. In this case, the behavior has been learned. As we will see, even neurologically simple organisms have a considerable capacity to learn. Fruit flies, for example, learn to avoid certain locations or substances if they associate them with an unpleasant experience.
We can consider, then, the extent to which a particular behavior is genetically encoded (part of the animal’s nature) and the extent to which it is conditioned by the environment in which the animal develops (part of the animal’s nurture). However, as we discussed in Chapter 18, nature and nurture are inextricably linked. For example, most human behaviors are the product of an interaction between nature and nurture. Consider language in the context of Tinbergen’s second question, concerning the development of behavior: We have an innate ability for acquiring language, but the specific language that we acquire depends entirely on our nurture. A baby in Italy learns Italian, and one in Finland learns Finnish.
45.2.1 The fixed action pattern is a stereotyped behavior.
Behavior can be complex and therefore influenced by a large number of factors. For that reason, complex behaviors can sometimes defy analysis. Scientists studying behavior tend to focus on simple behaviors because they are more easily studied and understood.

Some of the first behaviors to be carefully analyzed were displays, which are patterns of behavior that are species specific and tend to be highly repeatable and similar from one individual to the next. Presumably, natural selection has favored display behaviors that are unmistakable in their function as signals. Consider, for example, courtship displays that are often precursors to mating in birds (Fig. 45.2). Experiments have shown that a bird raised in complete isolation from other members of its species will still perform a courtship display with great precision.
Displays are an example of a fixed action pattern (FAP). A FAP is a sequence of behaviors that, once triggered, is followed through to completion. A classic example, originally studied by Tinbergen, is the response of a goose to an egg that has fallen from its nest (Fig. 45.3). The stimulus that initiates the behavior is the key stimulus and, in this case, it is the sight of the misplaced egg. This sight provokes in the goose an egg-retrieval FAP, which consists of rolling the egg back to the nest with the underside of its beak (Fig. 45.3a). This response cannot be broken down into smaller subunits and it is always carried out to the end, even if it is interrupted. In fact, the goose will persist in its efforts even if the researcher ties a string around the misplaced egg and removes it while the goose is in mid-action. The goose will still continue the task of rolling the now-absent egg (Fig. 45.3b).
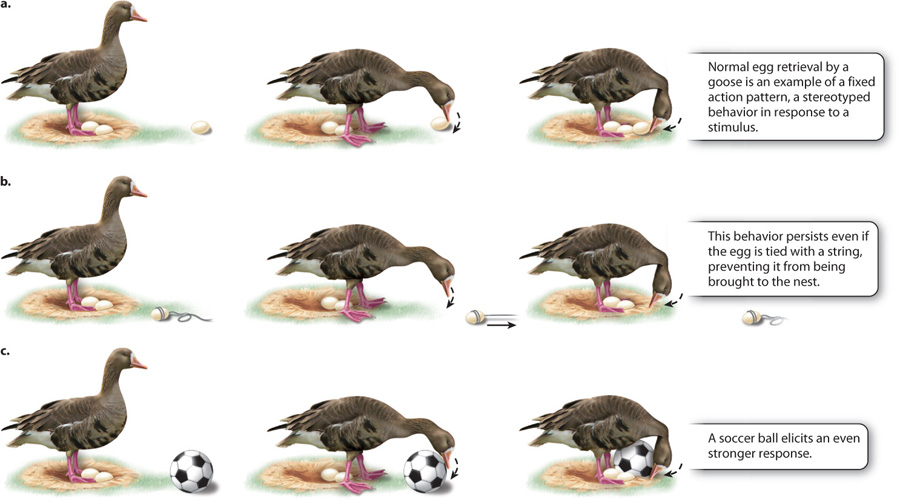
It is possible to understand this behavior by varying attributes of the key stimulus (in this case, the misplaced egg). The researcher can make models that vary in only one attribute to investigate how sensitive the goose is to aspects such as color or shape. A remarkable finding is that many birds respond most strongly to the largest round object provided, even a soccer ball, as illustrated in Fig. 45.3c. A soccer ball in fact not only elicits the egg-retrieval FAP, but it does so even more strongly than does a normal egg. The soccer ball is considered a supernormal stimulus because it is larger than any egg the goose would naturally encounter and elicits an exaggerated response. Natural selection has likely favored geese that recognize and respond to large eggs that have rolled outside the nest, but since eggs never grow to the size of soccer balls in normal circumstances, selection has not shaped an appropriate response to unrealistically large egg-shaped objects.
45.2.2 The nervous system processes stimuli and evokes behaviors.
How do animals recognize the stimulus that leads to a particular behavior? As we have seen, a goose attempts to retrieve an egg that is nearby but outside its nest. How does it recognize that the object in question is an egg? At its root, this is an extraordinarily difficult problem. The world is full of stimuli and there are many things that look like an egg. Thus, the challenge for the animal is to filter out the correct signal (the egg) from the surrounding noise (everything else). An animal has to process many different kinds of information in three dimensions.
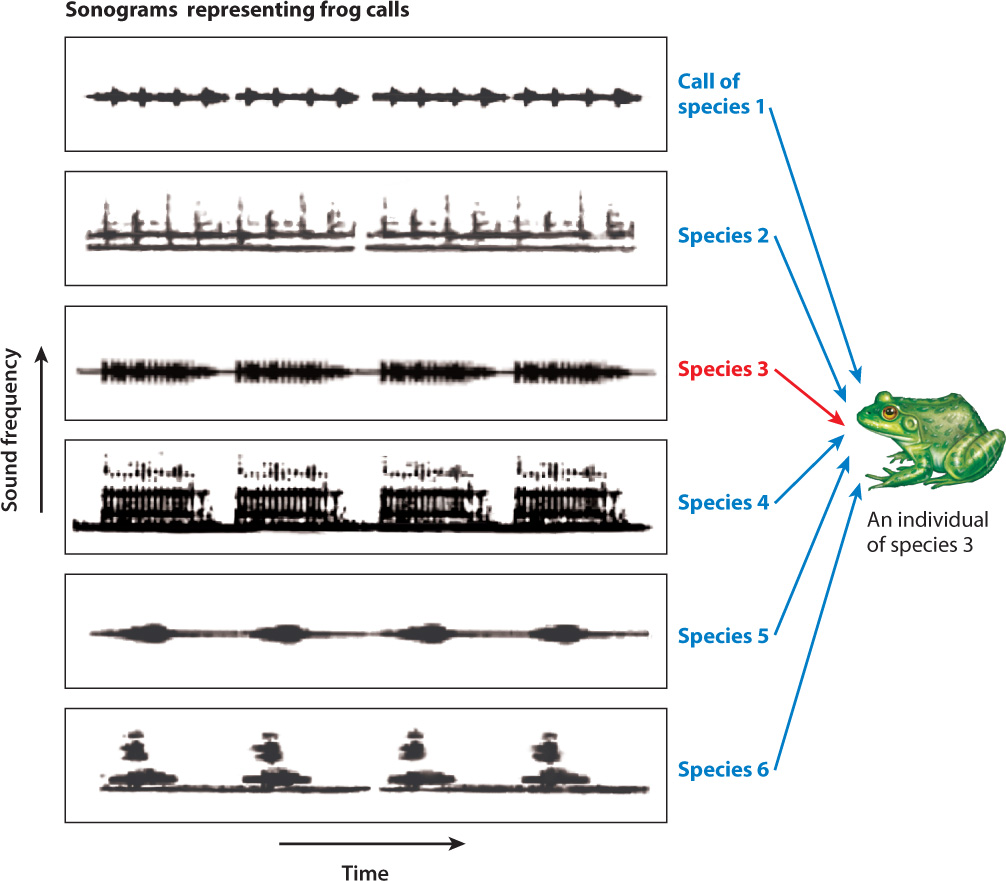
We know that stimulus recognition is often carried out by feature detectors, specialized sensory receptors or groups of sensory receptors that respond to important signals in the environment. Frogs provide a good example. Male frogs call to attract females and warn off other males. A single pond may contain many species of frog, each with its specific advertisement vocalization, or call. A heavily populated frog pond is positively cacophonous, but it is critical to an individual frog that it be able to distinguish the call of other members of its own species from those of other species. Further complicating the matter is that different species of frog can have similar calls, often virtually indistinguishable to the human ear.
One approach to the problem of understanding how frogs recognize their species-specific call has been to experiment with recordings of frog calls. By recording frog calls, altering aspects of the call in specific ways, playing them back, and then monitoring the responses of frogs, we can identify the impact of different components—pitch, duration, and pulse frequency, for example—of the call. These kinds of study have demonstrated that the frog’s auditory nerves can act as a feature detector, each one “tuned” to a specific component of the call, as shown in Fig. 45.4. The combination of different feature detectors stimulated by different components adds up to the recognition by the frog of a specific call. Once the sound has been correctly identified, the appropriate behavior follows.
45.2.3 Hormones can trigger certain behaviors.
As we have seen, stimuli are detected and processed by sensory receptors. In some cases, the response includes the release of a hormone. The power of a hormone lies in its ability to affect multiple cells in target organs simultaneously (Chapter 38). A neuron, on the other hand, is typically much more specific in its action, connecting directly to another neuron or neurons, or to a muscle (Chapter 35).
Hormones can affect behavior. The hormone testosterone, for example, is critical in sex determination in mammals, including humans (Chapter 42). In addition, in many respects males and females behave differently, and testosterone is at least partially responsible for many sex-specific behaviors.
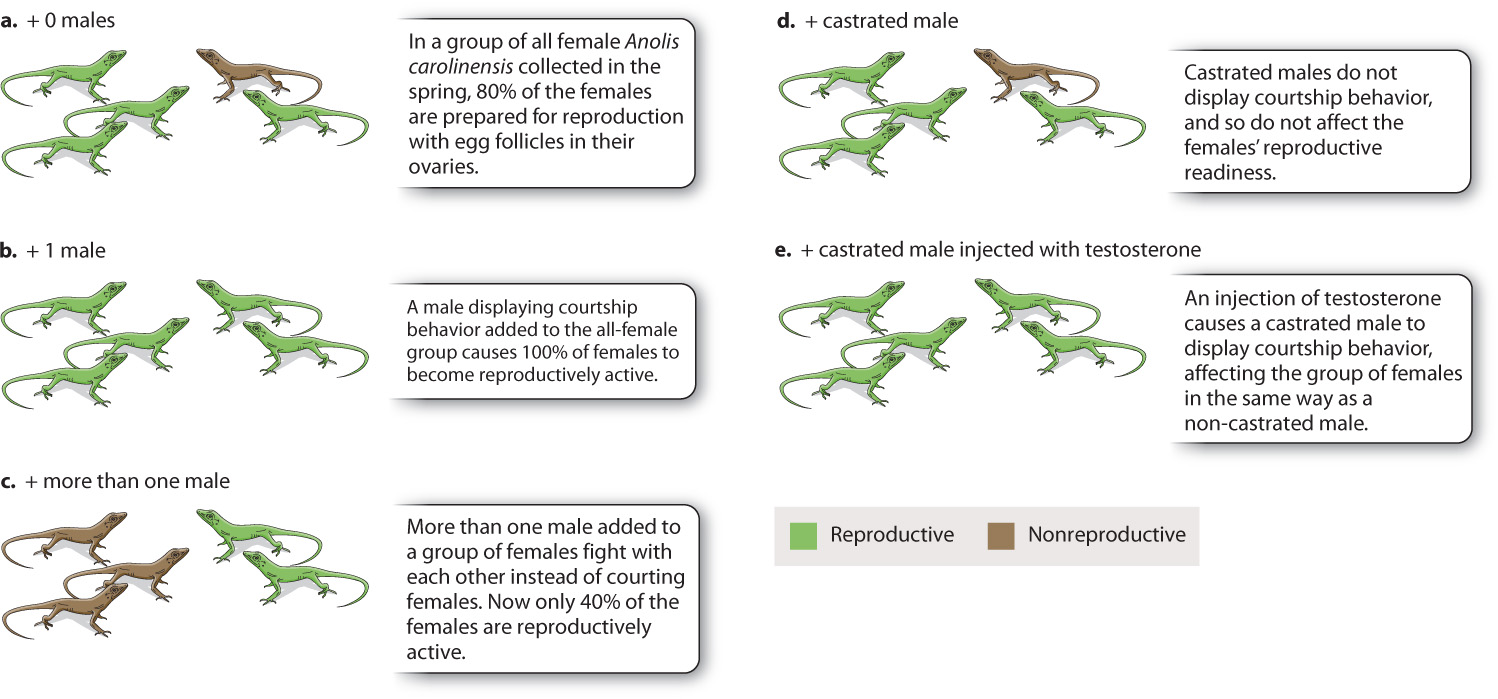
Anolis lizards demonstrate the important role of hormones in sexual behavior, showing both how social stimuli can affect the release of hormones and how hormones can affect behavior (Fig. 45.5). Females of Anolis carolinensis collected in the spring and housed in the laboratory under a spring light-dark cycle prepare for reproduction, and about 80% of individuals have active egg follicles in their ovaries (Fig. 45.5a). If one male is added to a group of females, however, that figure increases to 100% (Fig. 45.5b). The courting behavior of the male lizard stimulates the females to produce hormones that cause the full development of the ovaries, making the females reproductively active. If a group of males instead of a single male is added to an all-female population, on the other hand, only about 40% of the females undergo ovarian development (Fig. 45.5c). This unexpected result seems to occur because the males interact and fight among themselves rather than court the females, and therefore the courtship stimulus is lacking. Castrated males (which do not produce testosterone) that are added to a group of females have no effect on rates of ovarian development, which remains about 80% (Fig. 45.5d). They fail to court because the behavior is testosterone-mediated. However, a castrated male that is injected with testosterone does display courting behavior and has the same effect as the presence of a single male, inducing all the females to undergo ovarian development (Fig. 45.5e). These simple laboratory experiments demonstrate the complex interplay between hormones and behavior.
45.2.4 Breeding experiments can help determine the degree to which a behavior is genetic.
The nervous and endocrine systems that in part shape behaviors are the product of genetic instructions. If you take an extreme view, then, all behaviors have a genetic component. After all, it is the instructions in the genome that build the nervous system, muscles, and glands that lead to behavior. On the other hand, all behavior can be considered environmental since, without the appropriate environment, an organism would not develop the ability to perform the behavior. On a very basic level, an organism in an environment without appropriate nutrients would lack the necessary energy to perform a given behavior. How, then, do we study the genetic basis of behavior? Modern approaches harness the power of molecular genetics, but more traditional analyses are also highly informative.

Artificial selection, in which humans breed animals and plants for particular traits, provides strong evidence of the role of genetics in influencing behavior. Dogs were domesticated from wolves about 10,000 years ago. Today, there is great diversity among dogs, although they are all the same species. Obviously, there has been extensive selection on physical traits, with the result that a Dachshund, for example, looks quite different from a German Shepherd or Great Dane. Selection has also been applied to behavior: A Pointer has extraordinary “pointing” behavior that specifies the location of a hunter’s prey, and a Border Collie is an excellent herder (Fig. 45.6).
In the late 1950s, William Dilger studied the nest-building behavior of lovebirds in the genus Agapornis. Some species transport their nesting material in their beaks, whereas others tuck pieces into their tail feathers. Is there a genetic basis to the nesting behavior of these birds? To answer this question, Dilger set up crosses between species with different nest-building techniques to produce hybrids. He then observed how the hybrids built their nests. Interestingly, the hybrid offspring showed nest-building behavior intermediate between that of the parents: They tried to tuck material under their feathers, but were not successful. These experiments suggest that this behavior has a genetic basis.
It was not possible to do a true Mendelian dissection of the lovebirds’ behavior (Chapter 16). Such an analysis would require additional crosses, which were not possible because the hybrids were sterile. Like most behaviors, the lovebirds’ nest-building behavior is probably controlled by a large number of genes, each with a relatively small effect (Chapter 18). Therefore, it is difficult to identify which genes are responsible for the behavior. Even with molecular methods, the task of mapping and identifying the underlying genes when a large number of different genes govern a particular trait is daunting.
45.2.5 Molecular techniques provide new ways of testing the role of genes in behavior.
Molecular biology is changing the way we approach behavioral genetics. Some studies have identified complex behaviors that are strongly influenced by a single gene. One well understood instance of a single gene’s effect on behavior comes from the fruit fly Drosophila.
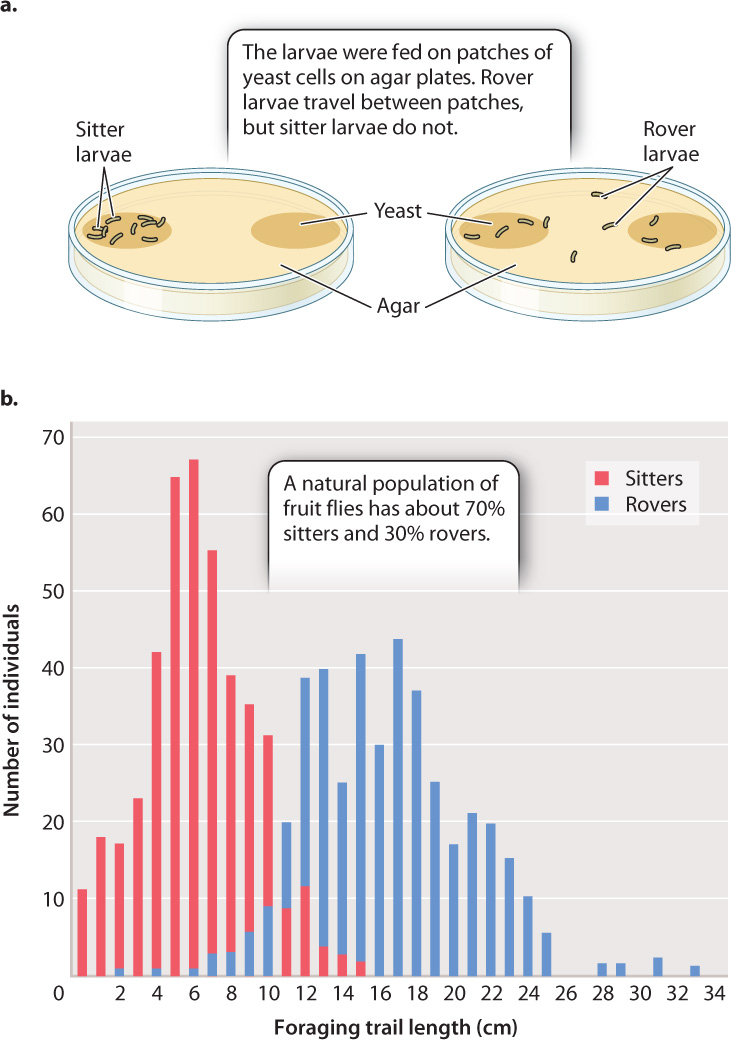
In Drosophila, there are two different alleles of the foraging (for) gene—fors and forR—that are common in populations. These two alleles have different effects on the behavior of Drosophila larvae. The gene encodes a cGMP-dependent protein kinase, an enzyme expressed in the brain that affects neuronal activity and alters behavior. In the absence of food, both “sitter” (fors) and “rover” (forR) larvae move about in search of food. In the presence of food, however, sitters barely move, feeding on the patch on which they find themselves, whereas rovers move extensively both within a patch of food and between patches (Fig 45.7a).
Both forms are present in natural populations (Fig. 45.7b); typically, 70% are rovers, 30% are sitters. This proportion suggests that both strategies are adaptive. Furthermore, studies have shown that rovers are selected for in crowded environments in which there is an advantage to seeking new food sources, and sitters are selected for in less-crowded ones because they take maximum advantage of their current food source. In this case, variation at a single gene affects a complex behavior in fruit flies.
The foraging gene is present in other insects as well, including honeybees, suggesting that it has been evolutionary conserved. Honeybees with low levels of for expression in their brain tend to stay in the hive, whereas those with high levels of for expression are likely to be foragers (Fig. 45.8).
FIG. 45.8: Can genes influence behavior?
BACKGROUND In fruit flies (Drosophila melanogaster), variation at the foraging (for) gene affects feeding behavior of larvae. Two alleles of the for gene—fors and forR—exist in natural populations; fors larvae tend to stay on a patch of food (“sitters”) and forR larvae tend to move from patch to patch (“rovers”). Honeybees (Apis mellifera) also forage for food, but, in contrast to fruit flies, this behavior changes with age. Young honeybees stay at the hive (“nurses”), and older ones forage for nectar (“foragers”). The for gene is present in honeybees, raising the possibility that this gene is involved in the developmental change in foraging in honeybees.
HYPOTHESIS Foraging in honeybees is influenced by the expression of the for gene, and nurses have lower expression levels than foragers.
EXPERIMENT 1 Levels of for mRNA were measured in honeybee nurses and foragers.
RESULTS Levels of for mRNA are significantly higher in foragers than in nurses (Fig. 45.8a). However, foragers are older than nurses, so it was unclear whether the increased expression in foragers compared to nurses is associated with differences in age or differences in behavior. Therefore, the experiment was repeated with honeybees that forage at a young age. These young foragers also have higher for mRNA levels than do nurses (Fig. 45.8b).
EXPERIMENT 2 The for gene encodes a cGMP-dependent kinase that phosphorylates other proteins (Chapter 9). Honeybee nurses were treated with cGMP to activate the kinase and their subsequent behavior was monitored. A related compound, cAMP, with a chemical structure similar to cGMP but which does not affect the kinase, was used as a control, to ensure that any effect observed was specific for the pathway and not the result of the treatment.
RESULTS Treatment with cGMP changed the behavior of nurses, causing them to forage (Fig. 45.8c). Furthermore, cGMP acted in a dose-dependent fashion: The higher the dose, the more foraging behavior was observed. No effect on foraging was seen in the control treatment (Fig. 45.8d).
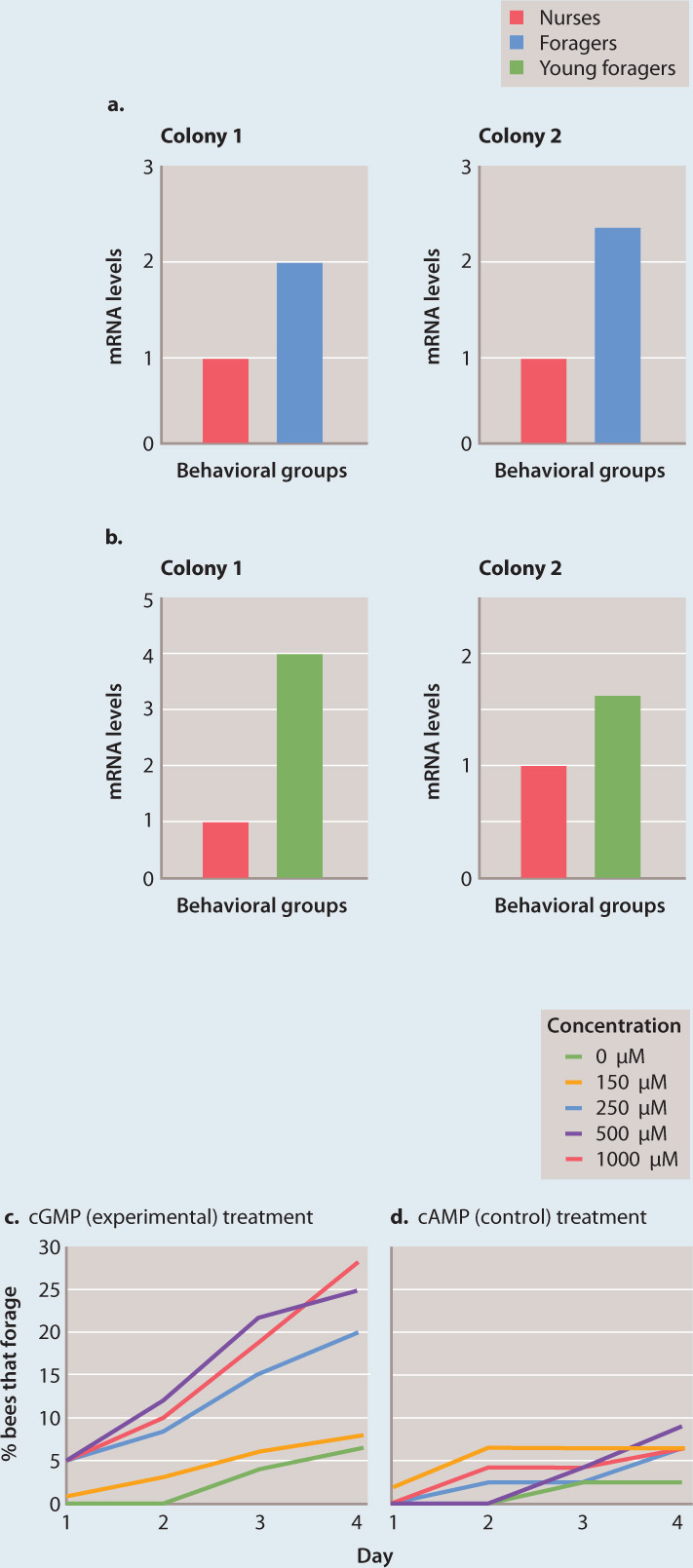
CONCLUSION The same gene that is involved in two different behavioral phenotypes in fruit flies (sitters vs. rovers) is also involved in a developmental behavioral change in honeybees (nurses vs. foragers). This finding suggests that a gene can have related but different functions in different organisms.
FOLLOW-UP WORK Researchers have looked at the expression profiles of many other genes in the honeybee genome to learn which genes are associated with the change of foraging behavior of honeybees.
SOURCE Ben-Shahar, Y., A. Robichon, M. B. Sokolowski, and G. E. Robinson. 2002. “Influence of Gene Action Across Different Time Scales on Behavior.” Science 296 : 741–744.
Question Quick Check 1
QfWDHvat2+Gf9SG6ca0bz3IEmdeCClIJWfMI4e6iL6yoTl/PCXuiBsN5XwKD5c7ySNH5Mr0feXQQN1PDCDTitpSANN0xSth0dv4B4CtI1rkRf8Cyl/h3GuNJq7DUXvuQdF5JC2Kh2vsxTOMDKoE3Xi8uPtfSQfGR/5YWX65APYjBGuXwATHU9v54+CY=Studies of the North American vole, in the genus Microtus, have also revealed how genes influence behavior (Fig. 45.9). The prairie vole is monogamous: Couples pair bond for life, meaning that they mate only with each other. By contrast, the prairie vole’s close relative, the montane vole, is promiscuous: A male mates and moves on, and a female, over her lifetime, produces litters by several different males. What differences underlie these different behaviors?

Hormones and their receptors provide an answer. In all mammals, antidiuretic hormone (ADH) controls the concentration of urine (Chapter 41). The receptor for ADH is not only expressed in the kidney, but also in the brain, where it affects behavior. Both the monogamous prairie vole and the promiscuous montane vole produce ADH, but the hormone receptors in the two species are different. The American neuroscientist Thomas Insel analyzed the genes that encode the ADH receptor in the two vole species. He found a difference in the region of the gene that determines under what conditions and in what cells it is expressed. Because of this difference, the distribution of ADH receptors in the brain is different in the prairie vole and in the montane vole.
Does the difference in gene expression explain the different sexual behaviors of the two species? The answer seems to be yes. Insel and his colleague Larry Young inserted the prairie vole ADH receptor gene, complete with its adjacent regulatory region, into a laboratory mouse (a promiscuous species, like the montane vole). Interestingly, Insel and Young observed a marked change in the mouse’s behavior. Rather than mating with a female and then moving on to another mate, the transgenic male mouse pair bonded with the female. In short, the addition of a single gene affected a complex behavior such as pair bonding, making the otherwise promiscuous mouse more likely to pair bond.