46.3 METAPOPULATION DYNAMICS
In nature, the populations of many, if not most, species are patchily distributed across the landscape. Deer occur only where there are forest patches of suitable size, milkweeds occur only in open fields, and ducks need to be near water. Local populations are therefore usually separated from one another in space and are connected only occasionally by individuals that migrate between habitat patches. Moreover, some habitat areas that could, in principle, support populations of a given species are unoccupied. How does this kind of distribution influence the dynamics—that is, the change in abundances—of populations in nature?
46.3.1 A metapopulation is a group of populations linked by immigrants.
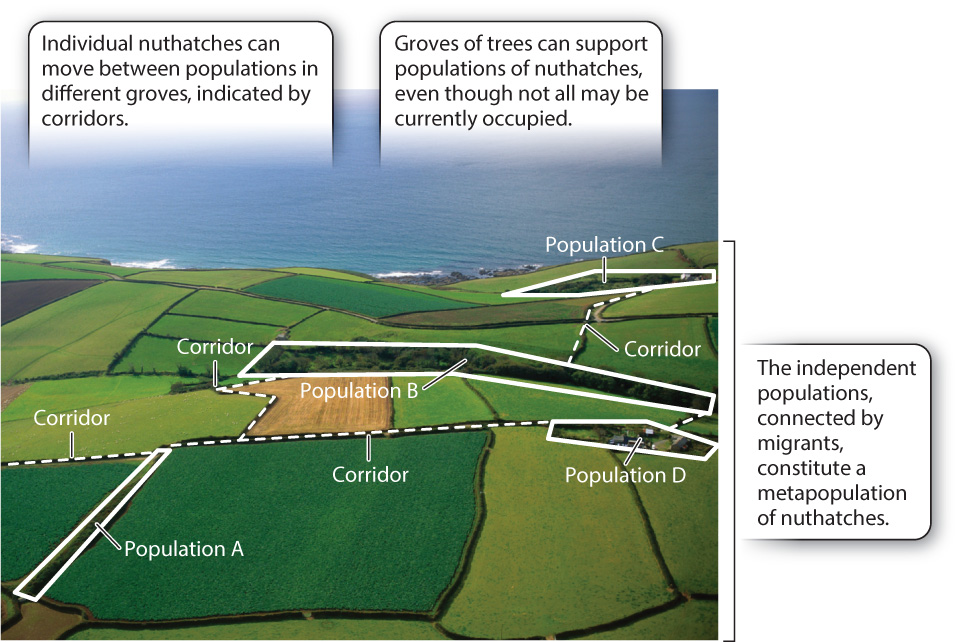
Groves of trees, separated from other groves by fields, are patches of habitat for the birds, insects, and fungi that rely on them for food and shelter. Similarly, humans are patches of habitat for infectious viruses and certain bacteria, coral reefs are patches for fish, and mountaintops are patches for mountain goats and other high altitude species. For nuthatches or bark beetles, crossing between groves of trees is a risky business, marked by predatory birds and the possibility of not finding another suitable grove. Similarly, populations of reef corals are often separated by open waters full of predatory fish. A patch, then, is a bit of habitat that is separated from other bits by inhospitable environments that are difficult or risky for individuals to cross.
The nuthatches that occupy a particular tree grove contact one another more frequently than they do individuals of other tree groves, and so each group is considered a local population. Occasionally, a few migrants might move from grove to grove, enabling gene flow between local populations. We can think of local populations of nuthatches in separate tree groves as each part of a larger, more inclusive group called a metapopulation. A metapopulation is a large population made up of smaller populations linked by migration (Fig. 46.13).
In order to understand the dynamics of populations, then, we must consider not only the birth and death of individuals, but also the colonization and extinction of local populations. Local populations within a metapopulation have independent fates and commonly become extinct because of their relatively small sizes. In general, small populations become extinct more often than large populations because they are more susceptible to random factors that increase mortality, such as weather, natural disasters, or predation. For example, a nest of predators can eliminate a local prey species; or, if population density falls too low in a small local population because of disease or lack of resources, survivors may not be able to find each other for mating. However, as long as some local populations remain—if they do not all become extinct at the same time—these populations can recolonize old habitats or find new habitat patches, thus ensuring survival of the metapopulation.
46-13
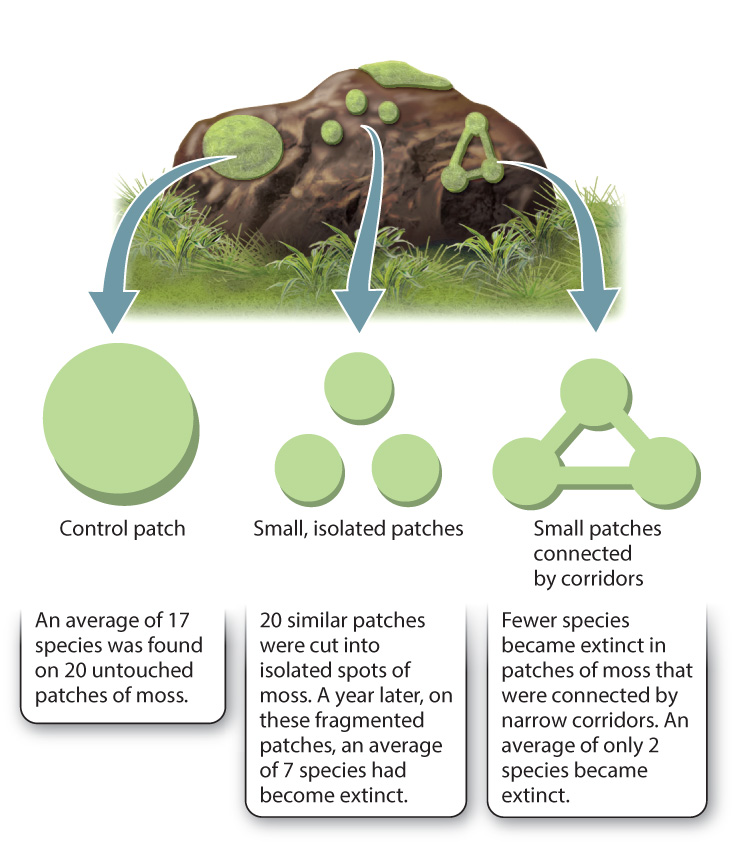
Experiments with patches of moss growing on boulders showed that the size and connectedness of the patches of moss affected the probability that species of arthropods inhabiting the moss would become extinct (Fig. 46.14). Researchers cut large patches of moss into several small fragments and sampled the number of insect species in each patch over time. As expected, isolated patches showed decreases in species diversity, up to 40% in 1 year; some species became extinct. However, when nearly isolated moss patches were left with a narrow corridor of moss connecting them, the rate of extinction was lower. Even narrow corridors of suitable habitat can allow species to move between patches of habitat and prevent extinction, or serve to reintroduce species that become locally extinct.

In a world increasingly marked by habitat fragmentation due to human activities, studies of metapopulations are playing an important role in conservation biology (Fig. 46.15). Species survival depends not only on the health of local populations, but also on the ability of individuals to colonize new habitat patches. As the simple experiment with mosses shows, corridors that connect endangered populations can dramatically increase the probability that a species will survive. However, corridors are important only to species that use them to cross barriers between patches. For species like ferns that produce spores able to float between patches on breezes, corridors may not be as important to patch colonization. Dispersal ability differs among organisms, strongly influencing their capacity to colonize distant patches.
46-14
46.3.2 Island biogeography explains species diversity on habitat islands.
Islands represent extremes in habitat patchiness for colonizing species—organisms that live on a mainland must successfully cross a barrier of water to establish a new population on an island. Ecologists commonly expand the use of the term “island” to include any habitat patch that is surrounded by a substantial expanse of inhospitable environment. While this use certainly includes parcels of land surrounded by water, it can also apply to a body of water surrounded by land, an expanse of forest surrounded by grasslands, or a mountaintop alpine field surrounded by forests at lower elevation. Where do colonists of these habitat islands come from, and what determines how many succeed in establishing a new population?
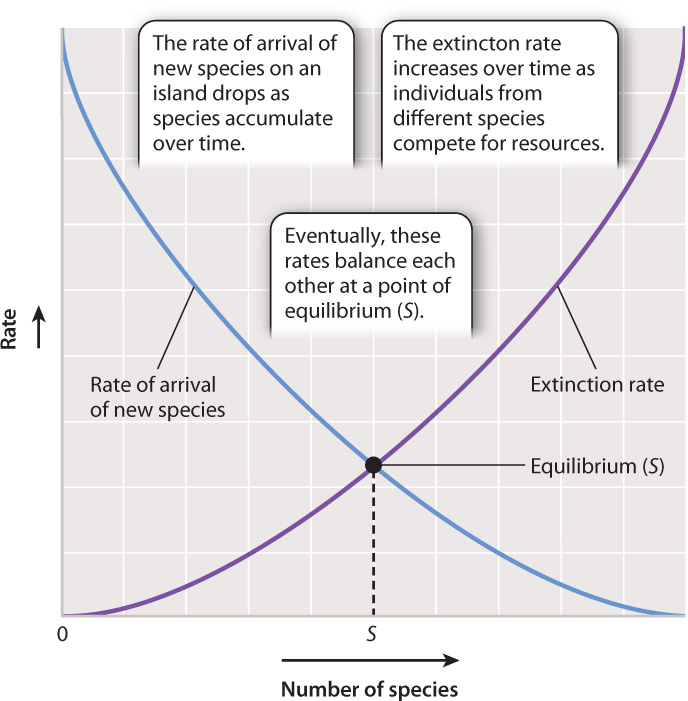
Species diversity reflects both the rate at which new species arrive on the island, and the rate at which species already on the island become extinct (Fig. 46.16). As species arrive on an initially uninhabited island, the colonization rate is high because there are no predators yet and the small number of colonists means that competition for resources is limited. Inevitably, as more and more species successfully establish themselves on the island, the rate of colonization goes down. This occurs both because competition and predation increase, making successful colonization more difficult, and because of the diminishing number of species left in the pool of potential colonists. In contrast, extinction rate goes up as newly arrived species compete.
At some point, there is a balance between the arrival of new species and the loss of species due to extinction. At this point, diversity is said to be at equilibrium (Fig. 46.16). This does not mean that no new species will colonize the island, but that each new colonist species will be accompanied by the extinction of one already in place. There is generally turnover in species composition through time.
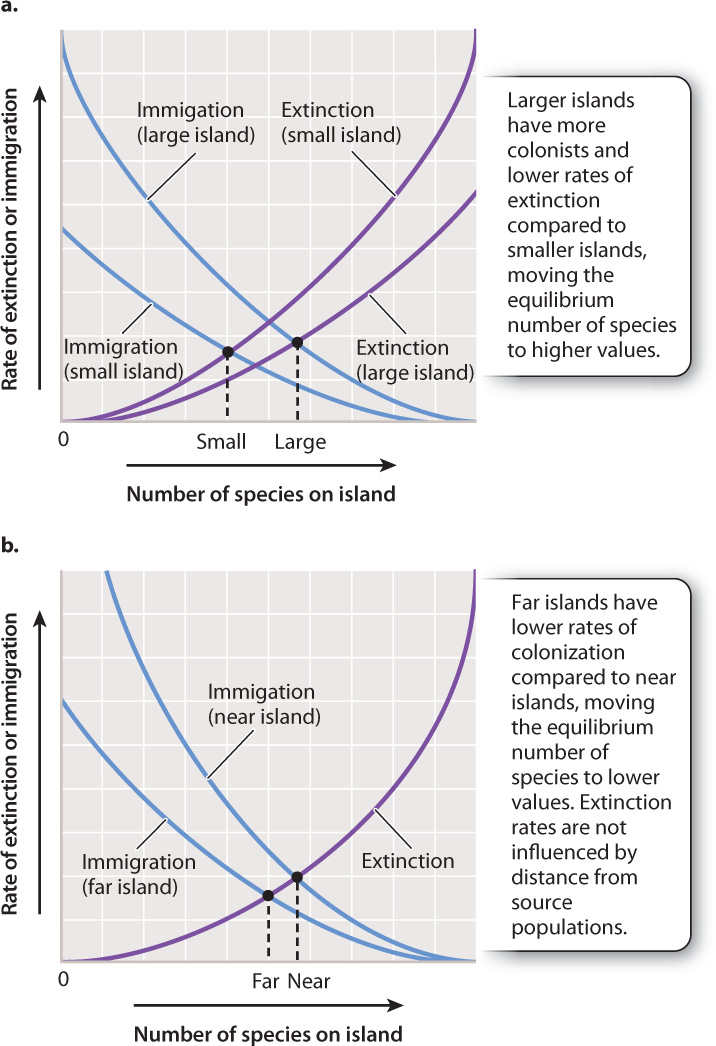
The theory of island biogeography, articulated in 1967 by the American biologists Robert MacArthur and E. O. Wilson, states that the number of species that can occupy a habitat island depends on two factors. The first is the size of the island. Because of their size, larger islands receive more colonists than smaller ones. Furthermore, larger islands can support more species than smaller ones can, so extinction rate goes down. For these reasons, the equilibrium number of species expected for larger islands will be greater than that for smaller islands (Fig. 46.17a). The second key factor is the distance of the island from a source of colonists. More distant islands have lower rates of colonization, moving equilibrium species diversity toward lower values (Fig. 46. 17b).
A number of observations and experiments have provided support for the theory of island biogeography. Wilson and his student Daniel Simberloff tested the theory of island biogeography in a novel way. Working among small mangrove islands off the Florida Keys, they used short-term insecticides to remove all the insects living on individual islands. Over the next few years, new insect populations established themselves on the mangrove islands. The researchers found that the species diversity of islands differing in size and distance from shore corresponded to those predicted by the theory. Larger islands had more successful colonists, whereas smaller and more distant islands had fewer.
At a larger scale, the number of animal species counted on tropical islands generally fits the predictions of island biographic theory. Fig. 46.18 shows not only that larger islands support more species than smaller ones, but also that there is a quantitative relationship between island size and species number. Similar relationships between island size and equilibrium species diversity have been observed repeatedly. The relationship can be described mathematically as a species–area relationship:
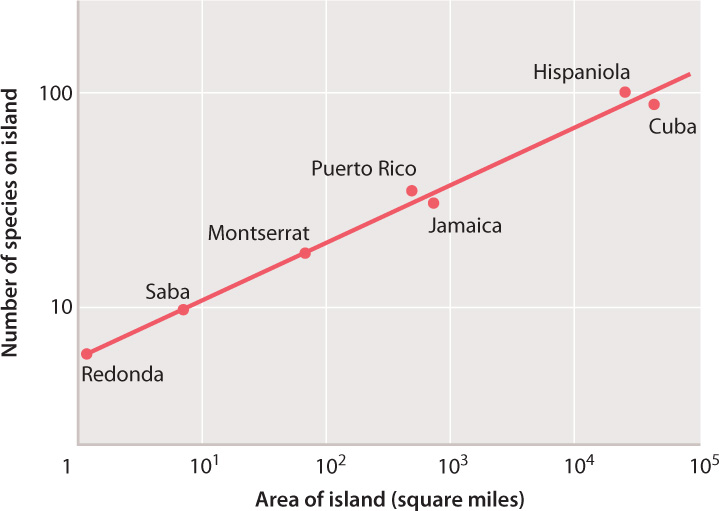
S = cAx
where S is the number of species at equilibrium, c is a mathematical constant, A is the habitat area, and x is an experimentally determined exponent, relating species number to habitat area. In many experiments, x has fallen within the narrow range of 0.1 to 0.4.
46-15
A useful back-of-the-envelope calculation is that a tenfold increase in island size results in a doubling of species diversity. Perhaps more relevant for issues of conservation, a 90% reduction of habitat area results in a 50% loss of species diversity if no other factors are taken into account. This relationship has many applications in conservation biology, among them the determination of how large a protected area must be to preserve biodiversity and how close reserves must be to one another to ensure the movement of at least some individuals between them.
Island biogeography theory has been widely applied, but it has some limitations in representing island colonizations over long evolutionary time frames. For example, the theory treats all potential colonists as equally able to colonize a new island, when in fact they are not all equally able to arrive or form an association with a species already in place. These abilities are often determined by a species’ particular evolutionary history. For example, flying animals like bats and birds more frequently arrive on oceanic islands than nonflying animals like rodents, lizards, and frogs. In contrast, the nonflying animals that colonize successfully more often evolve into multiple new species on islands.
Among the species that have colonized the island of Hispaniola, for example, there are many birds, bats, and strong flying insects like butterflies and dragonflies. Nonflying animals such as frogs, lizards, and fish became established by relatively few colonizations, but successful colonists diversified, evolving a larger number of closely related species now found nowhere else in the world; these are called endemic species.
Question Quick Check 5
7NZkSOm284BGRTqengTpdB38w5qPvvc3tZlQfLiv5gsaJ2XHdULzdq0khUmCP+y5kJLQ1hkpgcjm9uG9v8/LapCY+MLXRjt9augSzxcVrb61IzvGYe3Uj/6NvwRBAv5xymXTYpLFkEF/DGYEiVR7TjqC+7C+r/a4gAwE1J7g0j+vwxhki51ZQ0uDXEOeIjLn8U80Pw==46.3.3 How do islands promote species diversification?
Case 8 Biodiversity Hotspots: Rain Forests and Coral Reefs
Anolis lizards provide a classic and dramatic example of evolutionary diversification on the island of Hispaniola, where many dozens of species of closely related species coexist. Phylogenies suggest that Anolis lizards colonized Hispaniola in five or six separate events, but the dozens of species present today indicate marked evolutionary diversification after colonization.
46-16
All Anolis feed on insects and other invertebrates, but species differ in how and where they search for prey. Some with long legs run on the ground, while others with shorter legs and long tails climb on vegetation. Still other forms are large and robust and live in the high canopies of trees. Altogether, Anolis have evolved six different feeding strategies with corresponding morphological traits (Fig. 46.19).
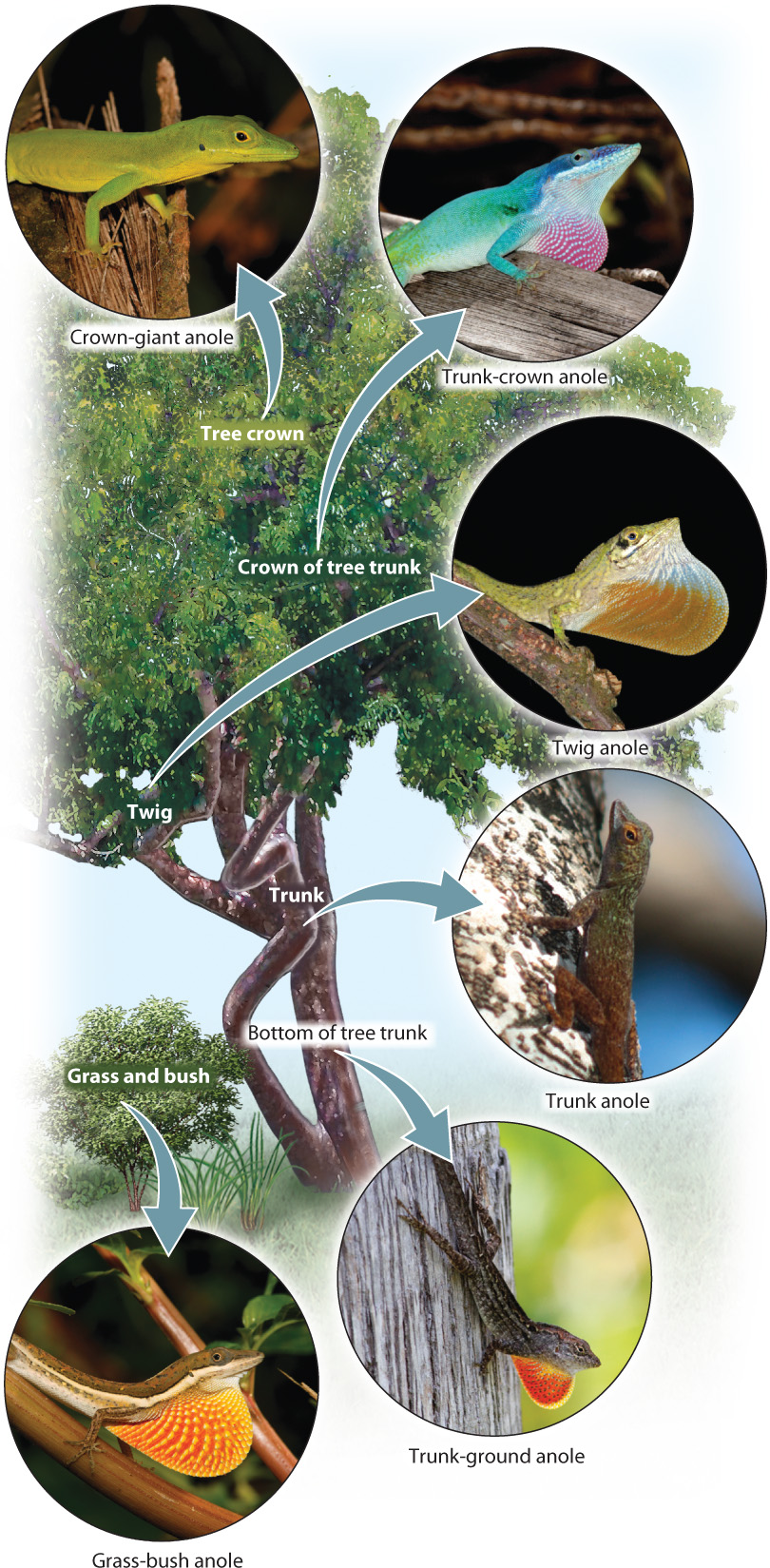
All six types can coexist in one place because they hunt insects in different ways and in different parts of the vegetation. Careful research is revealing the pattern of population expansion and fragmentation, followed by natural selection within different subpopulations, that has resulted in the diversity observed on Hispaniola today. Through evolution and complex ecological interactions, then, habitat diversity can depart from the simple predictions of island biogeography. Nonetheless, the theory remains a powerful way of thinking about species diversity and an important consideration in conservation biology.
46.3.4 Species coexistence depends on habitat diversity.
Species diversity on islands depends not only on the size of the island and its distance from the mainland, but also on interactions among species. Early experiments by the Russian biologist Georgii Gause showed that a simple system with one predator and one prey population is inherently unstable. The predator overexploits the prey, driving it to extinction, and then becomes extinct itself.
In 1958, the American scientist Carl Huffaker demonstrated that if the prey had refuges where some individuals could escape from predators, they could persist while predator populations declined. Through time, the prey population would recover and rise in population density to a point where predators would again expand and cause prey abundance to decline—and then decline again themselves (Fig. 46.20). Huffaker showed that predators and prey cycle repeatedly through periods of increasing and then decreasing density, as predators track their prey and some prey escape predation. In other words, a long-term, stable oscillation pattern can be achieved that links the population densities of predator and prey. We consider predator–prey interactions in more detail in the next chapter.
46-17
FIG. 46.20: Can predators and prey coexist stably in certain environments?
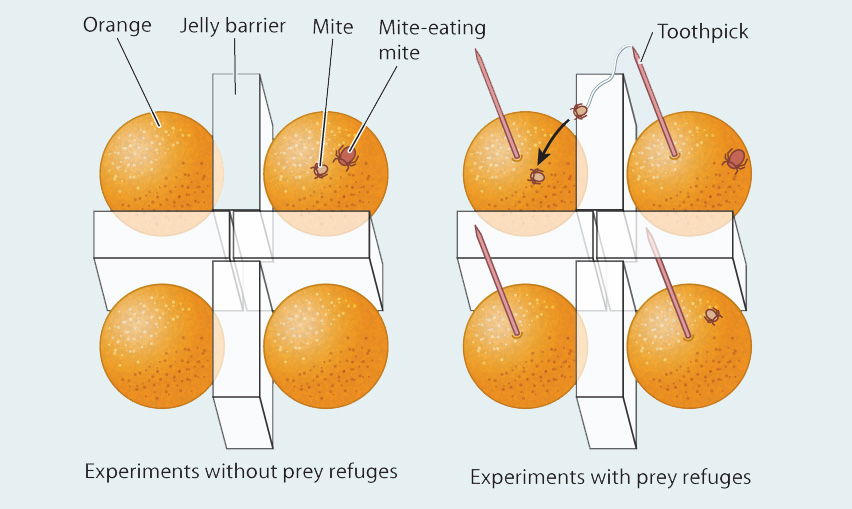
BACKGROUND In the 1950s, it was not clear whether a simple system of predators and prey could be stable and coexist indefinitely or whether it would become extinct as predators consumed all available prey. The results of experiments up to that time were equivocal, and ecologists knew that predators introduced to islands could hunt their prey to extinction.
HYPOTHESIS Ecologist Carl Huffaker hypothesized that predators and prey could stably coexist if temporary refuges were available for the prey.
EXPERIMENT Huffaker studied two kinds of mites: One feeds on the surface of oranges, and the other preys on the orange-eating mite. Huffaker put two sets of oranges on a table, separating them with barriers of petroleum jelly, essentially making each orange its own habitat patch. He added toothpicks to one set of oranges so that the orange-eating mites could let out silk and float over the petroleum jelly barriers and, at least temporarily, escape predation.
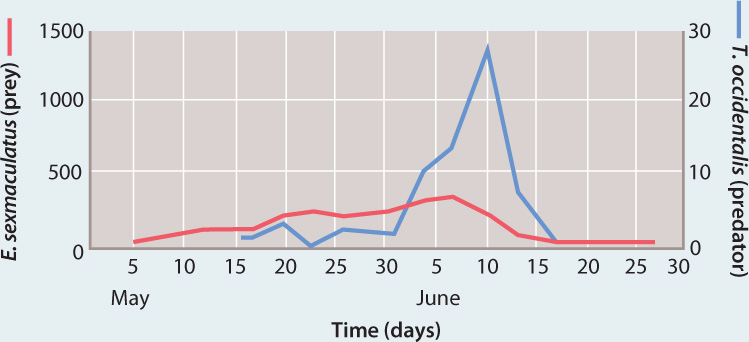
RESULTS In the setup in which prey could not escape, a single cycle of increase and decline was observed: The population size of prey increased but was closely tracked by rising populations of their predators, and eventually both declined to extinction.
In experiments in which toothpicks were provided, the orange-feeding mites climbed the toothpicks and dispersed on silken threads they produced, moving away from predators and toward other oranges without mites. These populations of predators and prey went through three cycles of increase and decline, and cycles would probably have continued if oranges continued to be supplied.
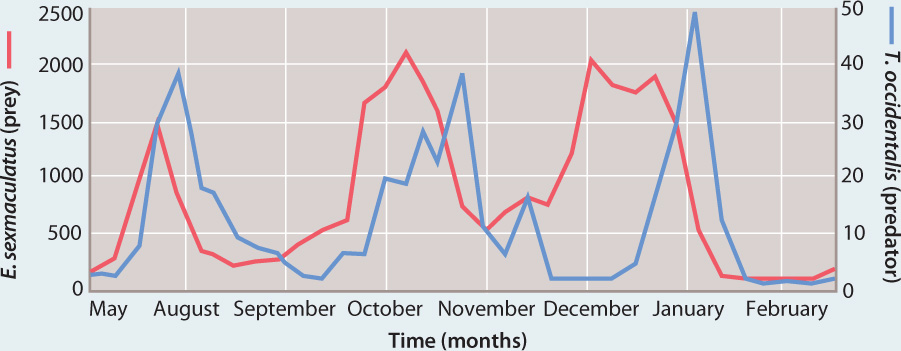
CONCLUSION Predator–prey systems can be stable if there are sufficient areas available where prey can escape predators, at least temporarily.
SOURCE Huffaker, C. B. 1958. “Experimental Studies on Predation: Dispersion Factors and Predator–Prey Oscillations.” Hilgardia: A Journal of Agricultural Science 27: 795–834.
46-18