47.4 ECOLOGICAL COMMUNITIES
We have been discussing how species interact and how these interactions can shape the distributions of populations, limiting distributions in one way or another. Now we look collectively at all the species that occur together in the same place. How, and to what extent, do they interact? And what are the properties of these spatial aggregations of species?
47.4.1 Species that live in the same place make up communities.
A community is the set of all populations found in a given place. That definition seems straightforward, but it raises some large questions about the nature of communities. Two different views were proposed nearly a century ago, and ecologists have debated their merits ever since. Frederic Clements, a prominent American plant ecologist of the early twentieth century, likened communities to a “superorganism” in which species interact strongly and predictably, like the organs within a body. In contrast, his contemporary, Henry Gleason viewed communities as simply the products of species acting individually in time and space. Not surprisingly, research inspired by their disagreement suggests that most communities lie somewhere between these extremes.
Populations in a community are tied together by the various interactions that secure their spot in a food web (Chapter 25), as well as by their physical location. But when we look at the details of where particular species occur, it turns out that almost no two species have exactly the same geographic distributions. This is partly a result of competitive exclusion (unless they are obligate associates of each other, like aphids and their bacteria). The Black Spruce and Bog Club Moss that inhabit freshwater bogs in northern New England overlap in their mutual preference for water-saturated, acidic, sandy soils found in cold temperate bogs, but they do not occur in the same places over most of their ranges.
Even within a local habitat like a meadow, species have different distributions that may reflect differences in soil moisture or patterns of sunlight exposure. For example, two kinds of milkweed occur in meadows, but one prefers damper sites and the other drier areas. For milkweeds and other plants, interactions also vary from place to place as the local set of species changes. Because a given species’ nearest neighbors differ over its geographic range, natural selection may also differ from place to place as particular competitors, partners, predators, or prey drop out or enter its distribution. For example, in the northern part of their range, Least Weasels prey only on lemmings, and so are under selection to coordinate their reproduction with that of the lemmings. But in the southern part of their range, lemmings are not present and so the weasels hunt mice and rabbits, placing different selection pressures on them.
The activities of different populations in a community also vary in time. For example, flies are active during the day, pollinating flowers that open in the morning and falling prey to dragonflies, birds, and other predators. In contrast, moths are important pollinators at night, when spiders and bats that prey on them are also active. Similarly, in coastal marine communities, oysters, crabs, and many other animals follow the daily rise and fall of tides. And, of course, the activities of many species change seasonally in responses to sunlight, temperature, and rainfall. Protein-rich insects that birds prey on to feed their young are most abundant in the spring, and fruits and seeds rich in the oils that migratory birds need to supply fat reserves are mostly available in late summer and fall.
47.4.2 A single herbivore species can affect other herbivores and their predators.
Predators and prey interact in settings as different as a tropical rain forest or Arctic tundra. In habitats with many different species of predators and prey, identifying the way each species affects others can be challenging. For this reason, much research on how predators and prey influence community structure has been carried out in the Arctic, where communities tend to have relatively few species.
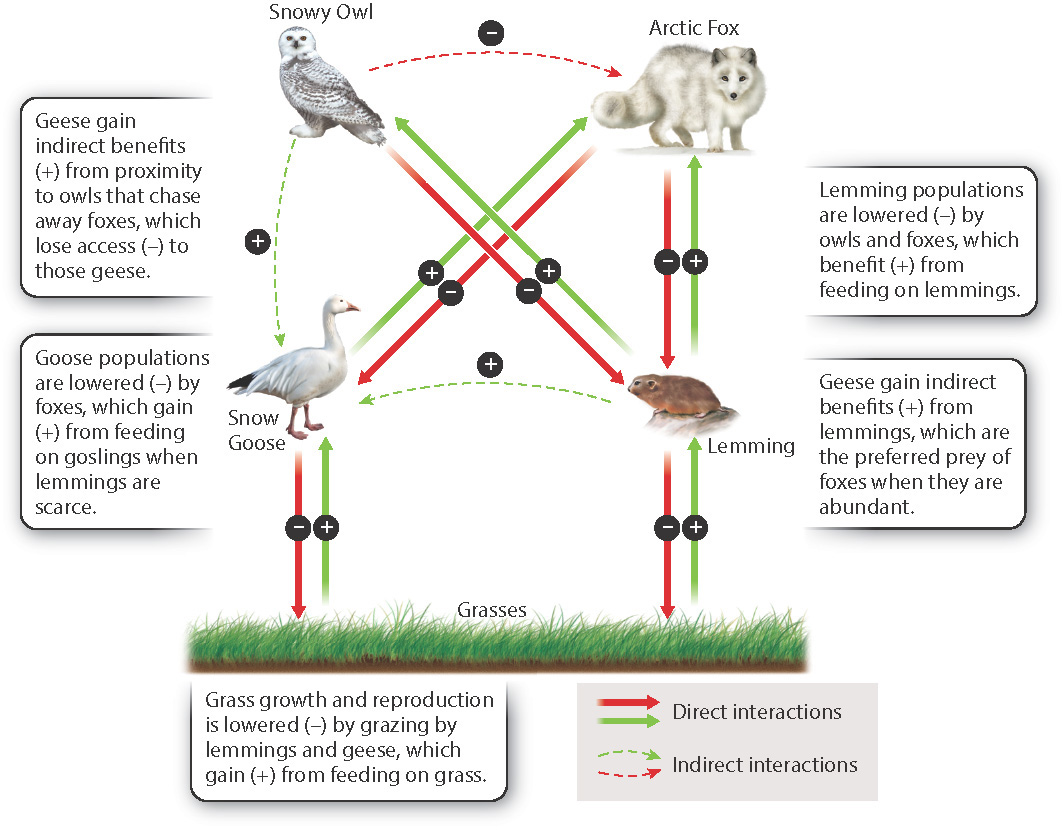
One well-studied community is found on Bylot Island in the Canadian territory of Nunavut. There, two species of lemming, the main herbivores in tundra communities, affect the populations of other herbivores and predators. Snow Geese are herbivores that live alongside the lemmings, and both geese and lemmings eat the grasses and other low plants that grow on the island. The main predators of lemmings and Snow Geese are Arctic Foxes and Snowy Owls. Lemmings, foxes, Snowy Owls, geese, and plants, then, are linked by a set of mutualisms and antagonisms, shown in Fig. 47.9. Changes in one species can affect all of the others.
Lemming populations rise and fall every 4 years or so. When lemming populations decline, foxes turn to other prey, including the eggs of Snow Geese (while the now harder-to-find lemmings escape and their populations begin to increase again). Snow Geese, therefore, are indirectly influenced by lemming population densities. The population density of foxes also reflects that of lemmings—as lemming populations increase to the point at which they can again become a significant part of fox diets, the foxes have access to enough resources to have more pups, increasing the fox population. In time, the additional predators again cause the lemming population to decline, and fox reproduction drops as a result. The population density of lemmings also influences grass abundance through its indirect effect on whether or not foxes eat Snow Goose eggs; young geese are heavy grazers in years when goose reproduction is high.
47.4.3 Keystone species have disproportionate effects on communities.
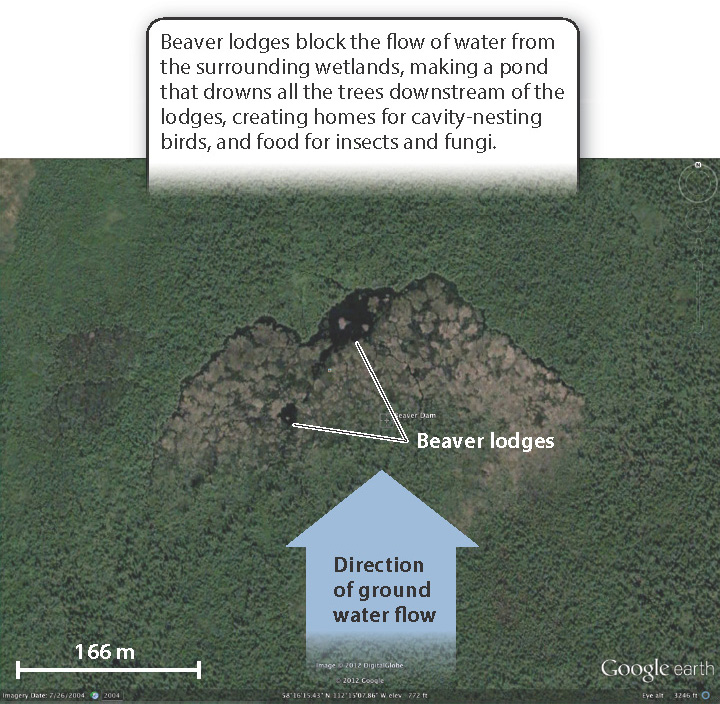
While all or nearly all species in a given place have some effect on one another, the integrity of a community may depend on a single species. This situation can occur when one species influences the transfer of a large proportion of biomass and energy from one trophic level to another or when the species modifies its physical environment, as beavers do when they make ponds. Such pivotal populations are called keystone species because they support a community in much the way a keystone supports an arch. Though all species contribute to the structure of a community, a keystone species affects other members of the community in ways that are disproportionate to its abundance or biomass.

A classic experiment by American zoologist Robert Paine gave birth to the concept of keystone species. Along the northwestern coast of the United States, mussels adhere to rocks in the intertidal zone, where they coexist with about two dozen other species of invertebrate animals and seaweeds. Mussel populations are regulated by their principal predator, a sea star called Pisaster ochraceus. When Paine removed the sea star from experimental plots, mussel populations exploded, outcompeting other animals and seaweeds for space and so eliminating them from the rocky shoreline. Control plots in which the sea star remained showed no such change. From these results, Paine concluded that the activities of the predatory sea star had a singularly strong influence on the diversity and distribution of other populations in the rocky intertidal community. Pisaster ochraceus is a keystone species.
Another experiment, this time unintended, cements the point. Along the west coast of the United States and Canada, giant kelp grow more than 50 m tall, providing a home for a remarkable diversity of fish and invertebrates (Fig. 47.10). The kelp community depends strongly on a single predator: the sea otter. Sea otters are the predators of sea urchins, which in turn graze on the rootlike holdfasts that anchor kelp to the seafloor. When the otters were removed by overhunting decades ago, sea urchin abundance soared and the kelp nearly disappeared, along with many other populations that live in kelp forests. Now, where otters are protected they once again keep urchin populations in check, enabling the kelp to return, and along with it, the diversity of fish and other animals for which kelp provides a habitat.
Keystone species called ecosystem engineers actively shape the physical environment, creating habitat for others. The classic example is beavers, which produce ponds by damming streams in forests, creating habitat that would not otherwise exist (Fig. 47.11).
Question Quick Check 3
YHvUJ6JhduaG0srhJLmw27haGEg3QhV7uKfhwkBiYCSBNplj8497NXQjUqOmrS/8TFgbv9Ya1j9M7aTKYDe7ParP1jLSKUKE0LbR7D9/jnA/sow180xEYi6IEr4=47.4.4 Disturbance can modify community composition.
We have seen how predation, herbivory, and parasitism can reduce the effects of competition. Severe climatic events, such as unusual rainfall or extreme temperatures, can also dramatically lower the abundances of some species, for example when an early frost kills mosquitoes and other insects. Severe physical impacts on a habitat, such as those caused by storms, earthquakes, or road building, are known as disturbances, and they have effects on populations of interacting species that are independent of their densities, as we saw in Chapter 46. Disturbances often affect multiple species in the same community and so can exert a strong influence over community composition. We usually think of disturbances as abiotic, but the line is not so clear with road-building by humans, for example, or dam-building by beavers. The main point is that catastrophic changes in a habitat affect populations in ways independent of their densities. Such changes are not uncommon, but are usually unpredictable.
Severe weather in forests demonstrates how disturbance can affect a community. When fire or a hurricane takes down tall trees in a forest, it also takes away the shade that had prevented some species from establishing new seedlings. Fires are frequent sources of disturbance in regions with a seasonally dry climate, such as parts of western North America and southwestern Australia. Like storms, fires remove incumbent plants and animals, opening up habitat for recolonization. Some species have, in fact, adapted to frequent fires. For example, several pine species have evolved thick bark and high branches, traits that help protect them from fire. Others, which have evolved cones that open only in the wake of fire, use the habitat space opened up by the fire and the nutrients that dead plant matter releases into the soil.
Disturbances allow species to enter or remain in the community as long as they can establish their young in the newly opened habitat patch. Noting the influence of storms on community composition in forests and coral reefs, American ecologist Joseph Connell proposed that the diversity of species in a given community reflects, at least in part, the frequency and intensity of disturbance. If disturbance is intense, few species can tolerate the physical conditions of the habitat and diversity will be low. On the other hand, if disturbance is rare or weak, competition may take over and only a few, stronger, competitors may remain. Species diversity tends to be highest when disturbance is frequent or intense enough to inhibit competition, but not so strong as to limit the number of species that can tolerate the environment.
47.4.5 Succession describes the community response to new habitats or disturbance.
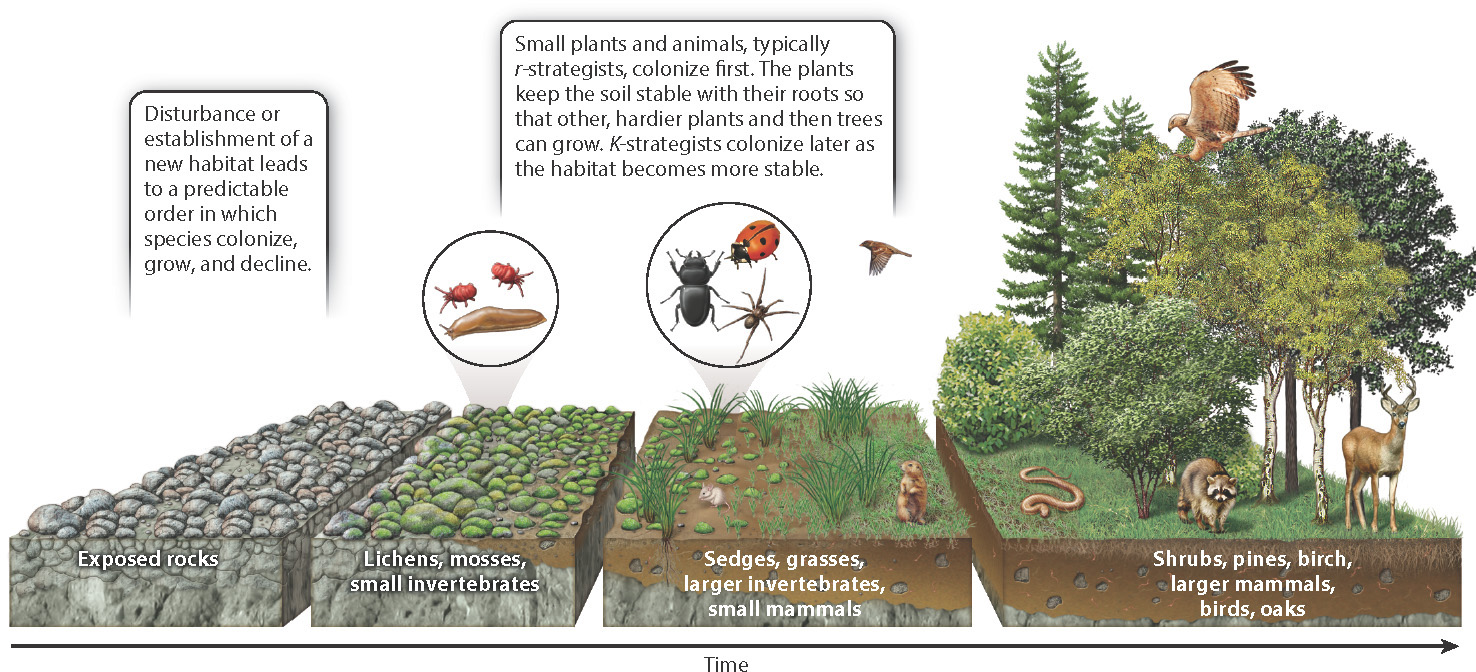
Following disturbance or the appearance of a new habitat, a community goes through a series of changes. These changes involve a predictable sequence of species that colonize and then transform the community, in what can appear to be a linear process of maturation. For example, following a large storm or fire, newly vacated habitat is commonly colonized by plant species adapted for rapid growth and strong sun. Eventually, these species are replaced by others able to grow in increasing shade. The pace of this transformation may be rapid, occurring over a few decades, or it may be much slower, depending on the environment.
This process of species replacing each other in time is called succession (Fig. 47.12). Clements viewed the pattern of species replacement in succession as strong evidence in support of his view that communities are highly integrated. Gleason, in contrast, explained succession purely in terms of the habitat preferences of individual populations.
The pin cherry, a resident of New England forests, illustrates how the life histories of individual species can contribute to succession. If you walk through a forest in New Hampshire, you will see oak trees and beeches, pine and hemlock. A mature forest may contain few or no pin cherry trees, but if you examine seeds lying dormant within the forest soil, you will see numerous pin cherry seeds. How did they get there?
Years earlier, disturbance from a storm or fire opened up the forest floor and pin cherries established themselves in the sunlit soils, growing from seeds left in the droppings of birds that had consumed the cherries elsewhere. The seedlings of pin cherries grow well in full sunlight, so the trees grew rapidly to reproductive maturity. A forest canopy was eventually reestablished, but pin cherry seeds do not germinate in the shade. In contrast, oak seedlings prosper in shade, so through time oaks and other species came to dominate this patch of forest. They will continue to dominate it until the next disturbance, when the removal of mature trees will once more expose the forest floor to bright sunlight, literally giving pin cherries another day in the sun.
The succession of species reflects the adaptations of each to the changes in sunlight, soil, water, and space that occur as the first organisms arrive, mature, and die. These organisms’ bodies influence their habitat in both life and death, whether aquatic or terrestrial. Succession over thousands of years can turn bare rock into forest. Lichens are often the first organisms to colonize bare rock newly formed by cooling lava or exposed by landslides. The lichens may start off with only a few mites and other tiny invertebrates living on them. As the stone weathers, and microorganisms feed on the lichen and one another, they form particles of soil that accumulate in cracks and crevices. Eventually, small plants, such as sedges and grasses, take root, and the growing plant community begins to gain height and diversity as other plants establish themselves.
Soon, larger insects, spiders, and other invertebrates join the community, as well as the first small mammals and birds. Many of these early colonizing species are r-strategists (Chapter 46), growing and reproducing quickly, and spreading their young far and wide. Birds carry the seeds of shrubs and other plants in their droppings, and the gradually deepening soil eventually permits the establishment of shrubs that in turn provide habitat for larger mammals and birds, and perhaps snakes and salamanders.
The first sun-loving trees, such as birches and pines, appear next, providing shade for the seedlings of later colonizing trees like oaks and maples. In many forests, these stable, long-lived K-strategists (Chapter 46) make up a final stage in the succession, forming a mature assembly commonly called a climax community. A climax community is one in which there is little further change in species composition.