Chapter 1. Molecular Geometry, Bonding, and Polarity
Discussion
Objectives

At the end of this activity you should be able to:
- Write Lewis structures for molecules.
- Classify bonds as nonpolar covalent, polar covalent, or ionic based on electronegativity differences.
- Recognize exceptions to the octet rule; draw accurate representations.
- Describe three-dimensional shapes of simple molecules based on VSEPR theory.
- Predict polarity based on geometry and individual dipole moments.
These learning objectives are assessed in the post-lab quiz.
There is no laboratory report.
Introduction
The substances in our world exhibit remarkably different properties. At room temperature some substances are solids, others liquids, and others gases. Some participate in sudden chemical reactions, whereas others are quite inert and unreactive. Perhaps most remarkably, this wonderful diversity occurs even though the substances are comprised of a limited number of elements. Indeed, only a very small number of different elements are present in almost any pure substance we encounter in the environment or the laboratory. How can this wide diversity of properties be explained?
A key to understanding the wide range of physical and chemical properties of substances is recognizing that atoms combine with other atoms to form molecules or compounds and that the shape or geometry of a collection of atoms strongly affects the properties of that substance. One reason this occurs is because the distribution of charge in a molecule affects many properties of the substance. For example, if the negative change is concentrated in one region of a molecule, its properties will be widely different than if the charge is distributed evenly throughout the entire molecule.
In this investigation, you will examine a theory that chemists use to explain different aspects of chemical bonding: Valence shell electron pair repulsion (VSEPR) theory. Attention will be given to how molecules are arranged in different shapes and how chemists can predict the geometry of a given molecule. It will then be shown how a molecule’s shape, along with electronegativity differences for its atoms, determine the molecule’s polarity. As suggested above, the best way to understand and predict the physical and chemical properties of substances in our world is by understanding their structure at the molecular level.
Discussion of Activities
In this investigation, you will complete activities that ask you to examine molecular geometry and molecular polarity. These activities are based on computer simulations and balland-and-stick models. All of these activities can be discussed with classmates and/or completed in small groups. Your teaching assistant will check on your progress.
The VSEPR model can be used to predict the geometry of molecules and polyatomic ions. Molecular geometry describes the positions of the nuclei in relation to each other. Included in the description are the bond angles—the angles made by the lines joining the nuclei of bonded atoms. In order to predict geometry using the VSEPR model, we need to know the number of electron pairs in the valence shell of the central atom. That can easily be determined by drawing a Lewis structure.
Activity 1: Drawing Lewis Structures
Guidelines for Drawing Lewis Structures
- Determine the total number of valence electrons; for polyatomic ions, remember to adjust for charge.
- Arrange atoms in a skeleton structure and connect them with single bonds.
- If you are working with a molecule or ion that has three or more atoms, the least electronegative atom is most likely to be the central atom (remembering that hydrogen can only form one bond and therefore is never a likely candidate to be the central atom).
- Complete octets of the terminal atoms (remember H atoms can only accommodate two electrons).
- If not all of the valence electrons have been used place any extra electrons on the central atom.
- Extra electrons placed on the central atom may in some cases bring the number to more than eight. This is called expansion of the valence shell and is an exception to the octet rule; this is acceptable for atoms in the third period and below.
- If the central atom does not have an octet, use lone pairs from terminal atoms to form multiple bonds.
- Only the second period elements C, N, O, and sometimes S (in combination with C or O) form multiple bonds. This leads to another exception to the octet rule, e.g., when an atom like Be is combined with either hydrogen or halogens, as in BeH2. Since Be does not form multiple bonds, the central atom is electron-deficient.
If more than one acceptable Lewis structure can be drawn by simply choosing a lone pair from a different terminal atom to form a double bond with the central atom, the different structures are called resonance forms. The “extra” electron pair is delocalized—spread out among the possible bonding sites.
Table 10.1 Drawing Lewis structures and determining electron and bonding domains.
| Sketch of Lewis Structure |
Does the Structure Violate the Octet Rule? |
Number of Electron Domains (central atom) |
Number of Bonding Domains (central atom) |
|
|---|---|---|---|---|
| NH3 | ||||
| H2O | ||||
| BF3 | ||||
| CO2 | ||||
| XeF6 |
One reason Lewis structures are useful is because they help to identify the number of electron domains, or regions of high electron density, about a central atom. An electron domain can be a bonding pair, a lone pair, or a double or triple bond. A multiple bond is counted as one domain.
The Lewis structure for HCN follows. Notice that the central carbon atom has two electron domains (a single bond and a triple bond) and also two bonding domains. The nitrogen atom also has two electron domains (a lone pair of electrons and a triple bond) but only one bonding domain. The hydrogen atom only has one electron domain (the single bond) and one bonding domain.
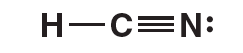
Activity 2: VSEPR and Predicting Molecular Geometry
Once we have the Lewis structure, we have the information needed to predict the geometry. It’s important to remember that what we really want to know is the molecular geometry—the positions of the nuclei in relation to each other. The molecular geometry is dependent on the electron domain geometry; that is why the initial step is drawing the appropriate Lewis structure! As noted above, the simple concept behind valence shell electron pair repulsion theory (VSEPR) is the idea that electron pairs in the valence shell of an atom will repel each other and arrange themselves as far apart as possible. This arrangement of electron pairs will determine the geometry of the molecule or polyatomic ion.
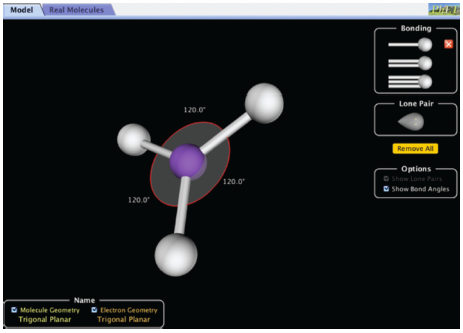
Your initial task in this activity is to determine the molecule geometry as the number of electron pairs changes. Accomplish this by using the computer simulation “Molecule Shapes” (shown in Figure 10.1) and fill in the table.
Note: In this simulation you can increase the number of electron domains by adding single, double, or triple bonds, or lone pair electrons. In the lower left corner you will find both the molecule geometry and the electron geometry.
Table 10.2 Model electron domain geometries.
| Number of Electron Domains |
Electron Domain Geometry |
Bond Angles |
|---|---|---|
| 2 | Linear | 180° |
| 3 | ||
| 4 | ||
| 5 | ||
| 6 |
The molecular geometry is the same as the electron domain geometry if all domains contain bonding pairs. What if lone pair electrons are present on the central atom? The molecular geometry will not be the same as the electron domain geometry. To investigate this, return to the simulation and complete Table 10.3. If you do not understand the names for the different geometries, ask your teaching assistant.
Table 10.3 Electron and molecular geometries.
| Number of Electron Domains |
Bonding Pairs |
Nonbonding Pairs |
Electron Domain Geometry |
Molecular Geometry |
|---|---|---|---|---|
| 2 | 2 | 0 | ||
| 3 | 3 | 0 | ||
| 3 | 2 | 1 | ||
| 4 | 4 | 0 | ||
| 4 | 3 | 1 | ||
| 4 | 2 | 2 | ||
| 5 | 5 | 0 | ||
| 5 | 4 | 1 | ||
| 5 | 3 | 2 | ||
| 5 | 2 | 3 | ||
| 6 | 6 | 0 | ||
| 6 | 5 | 1 | ||
| 6 | 4 | 2 |
Question 10.1: VSEPR stands for “valence shell electron pair repulsion.” How does electron pair repulsion determine the molecular geometry? Discuss two specific examples from the table. (Hint: In the simulation, is it possible to force the electron domains to be close together?)
Question 10.2:
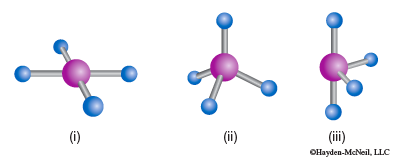
- For each shape identify the molecular geometry.
a.
b.
c.
- Determine the electron domain geometry on which the molecular geometry is based.
a.
b.
c.
- How many lone pairs are there on each central atom?
a.
b.
- Which of the following element(s) could be the central atom in shape (c)?
Be, C, S, Se, Si, Xe?
You may have noticed that some electron domains appear larger than others. To investigate how this may affect the resulting molecular geometry, complete Table 10.4 and predict both the geometry and the bond angles. Then, compare your predictions with the experimentally determined bond angles (as shown in the simulation under the tab Real Molecules).
Table 10.4 Comparison of electron domains.
| Molecule | Lewis Structure | Predicted Molecular Geometry | Predicted Bond Angles | Experimentally Determined Bond Angles |
|---|---|---|---|---|
| NH3 | ||||
| H2O | ||||
| SO2 |
Question 10.3: Which assumption about the space occupied by nonbonding (lone pair) electron pairs is most consistent with the experimental bond angles: Do nonbonding pairs occupy more, less, or the same amount of space as bonding pairs?
1.1 Activity 3: Molecular Polarity
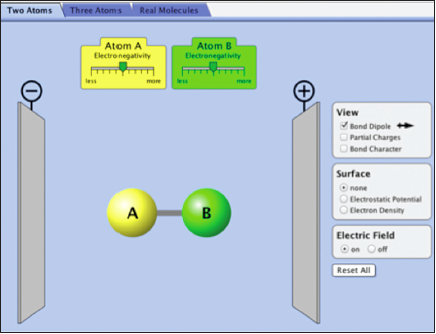
Investigating Bond Polarity with the Molecule AB
Select the “Two Atoms” tab on the top.
In this simulation, you will examine the bond polarity, which is a measure of how equally the electrons in a bond are shared between the two atoms of the bond.
There are different ways of representing where the electrons are in a bond. Take a few minutes and try the different “views” in the simulation, i.e., bond dipole, partial charges, and bond character. The electron density or electrostatic potential are other ways of communicating where the electrons are in the molecule.
Note: Within the simulation you change the relative electronegativity of atoms A and B.
Question 10.4: Explain how the direction of the arrow in the bond dipole symbol

relates to the electron density and the partial charges.
How does changing the electronegativity of the atoms affect the bond polarity?
How does changing the electronegativity of the atoms affect the bond character?
Molecules in Electric Fields
In this simulation, the molecule AB is placed between electric plates (–) and (+) and an electric field can be turned on or off. It is also possible to click on the molecule AB and rotate it.
Question 10.5:
| a. | What happens to the molecule AB when it has a bond dipole and the electric field is turned on? Spin the molecule around several times and make observations. What if the bond dipole is zero? |
| b. | Is there a relationship between the magnitude of the bond dipole and how the molecule is affected by the electric field? |
Investigating Bond Polarity and Molecular Polarity with the Molecule ABC
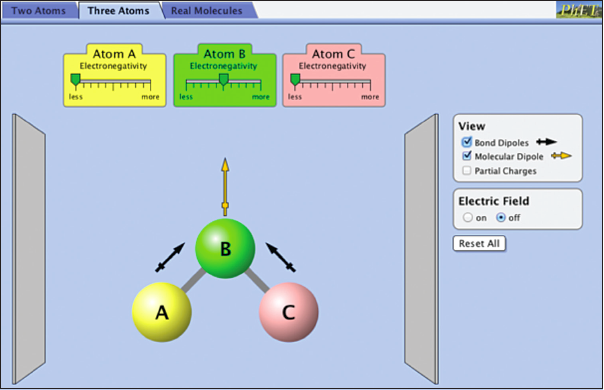
Select the Three Atoms tab on the top.
In the view section a new option has been added: “Molecular Dipole.” Molecular polarity describes the charge distribution in the entire molecule, not just a bond. If centers of positive and negative charge do not coincide, the molecule is polar. How can you predict if a molecule is polar? The two important variables are 1) the bond dipoles in the molecule, and 2) the molecular geometry.
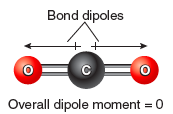
It is important to note that bond dipoles are vector quantities; that is, they have both a magnitude and a direction. In a polyatomic molecule, like ABC, the magnitude and the direction of the individual bond dipoles must be considered when summing vectors. As an example, consider the molecule CO2. In this molecule there are two bond dipoles, because the electronegativity of carbon and oxygen differ. There is no overall molecular dipole, however, because the bond dipoles “cancel” since they are of equal magnitude and pointed in opposite directions. Carbon dioxide is an example of a nonpolar molecule that has polar bonds.
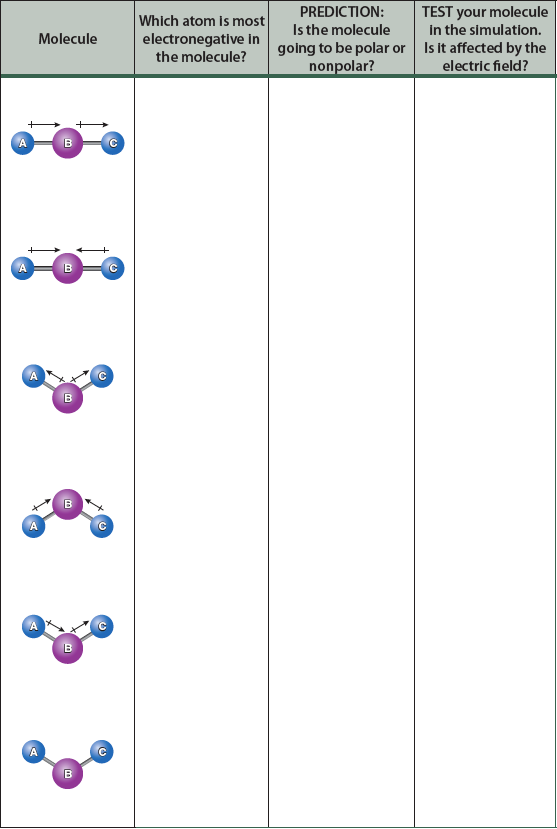
To explore the idea of molecular polarity, complete the following table. Make an initial prediction as to whether each ABC molecule will be polar or nonpolar based on the bond dipoles and the geometry; remember, bond dipoles are vector quantities. Then, construct the molecule in the simulation and see how it behaves in the electric field. Were your predictions correct?
Putting It All Together
Being able to predict the polarity of a molecule is extremely important since many properties of molecules depend on whether they are polar or nonpolar. As you have seen in this activity, determining a molecule’s polarity is a multi-step process:
Draw Lewis structure
↓
Use VSEPR to determine molecular geometry
↓
Determine bond polarity (based on electronegativity differences)
↓
Determine molecular polarity based on bond dipoles and molecular geometry
For the following molecules, complete this step-by-step process.
| Molecule | Lewis Structure | Molecular Geometry | Is there a molecular dipole? A molecular dipole indicates it is a polar molecule. |
|---|---|---|---|
| N2 | |||
| H2O | |||
| BF3 | |||
| HCN | |||
| CH2F2 |
Make a prediction, then check it in the “Real Molecules” section of the simulation.