13.2 Physical Effects of Stress: THE MIND–BODY CONNECTION
KEY THEME
Stress affects physical health through its effects on the endocrine system, the immune system, and chromosomes.
KEY QUESTIONS
What endocrine pathways are involved in the fight-or-flight response and the general adaptation syndrome?
What are telomeres, and how are they affected by acute and chronic stress?
What is psychoneuroimmunology, and how does the immune system interact with the nervous system?
From headaches to heart attacks, stress contributes to a wide range of disorders, especially when it is long-term, or chronic. Basically, stress appears to undermine physical well-being in two ways: indirectly and directly.
First, stress can indirectly affect a person’s health by prompting behaviors that jeopardize physical well-being, such as not eating or sleeping properly (Habhab & others, 2009; Mezick & others, 2009). High levels of stress can also interfere with cognitive abilities, such as attention, concentration, memory, and decision making (McNeil & Morgan, 2010; Thompson, 2010). In turn, such cognitive disruptions can increase the likelihood of accidents and injuries.
Second, stress can directly affect physical health by altering body functions, leading to physical symptoms, illness, or disease (Zachariae, 2009). For example, people who are under a great deal of stress often tighten their neck and head muscles, resulting in tension headaches. But exactly how do stressful events influence bodily processes, such as muscle contractions?
Stress and the Endocrine System
To explain the connection between stress and health, researchers have focused on how the nervous system, including the brain, interacts with two other important body systems: the endocrine and immune systems. We’ll first consider the role of the endocrine system in our response to stressful events and then look at the connections between stress and the immune system.
WALTER CANNON: STRESS AND THE FIGHT-OR-FLIGHT RESPONSE

Any kind of immediate threat to your well-being is a stress-producing experience that triggers a cascade of changes in your body. As we’ve noted in previous chapters, this rapidly occurring chain of internal physical reactions is called the fight-or-flight response. Collectively, these changes prepare us either to fight or to take flight from an immediate threat.
fight-or-flight response
A rapidly occurring chain of internal physical reactions that prepare people to either fight or take flight from an immediate threat.
The fight-or-flight response was first described by American physiologist Walter Cannon, one of the earliest contributors to stress research. Cannon (1932) found that the fight-or-flight response involved both the sympathetic nervous system and the endocrine system (see Chapter 2).
With the perception of a threat, the hypothalamus and lower brain structures activate the sympathetic nervous system (see left side of FIGURE 13.4 below). The sympathetic nervous system stimulates the adrenal medulla to secrete hormones called catecholamines, including adrenaline and noradrenaline. Circulating through the blood, catecholamines trigger the rapid and intense bodily changes associated with the fight-or-flight response. Once the threat is removed, the high level of bodily arousal subsides gradually, usually within about 20 to 60 minutes.
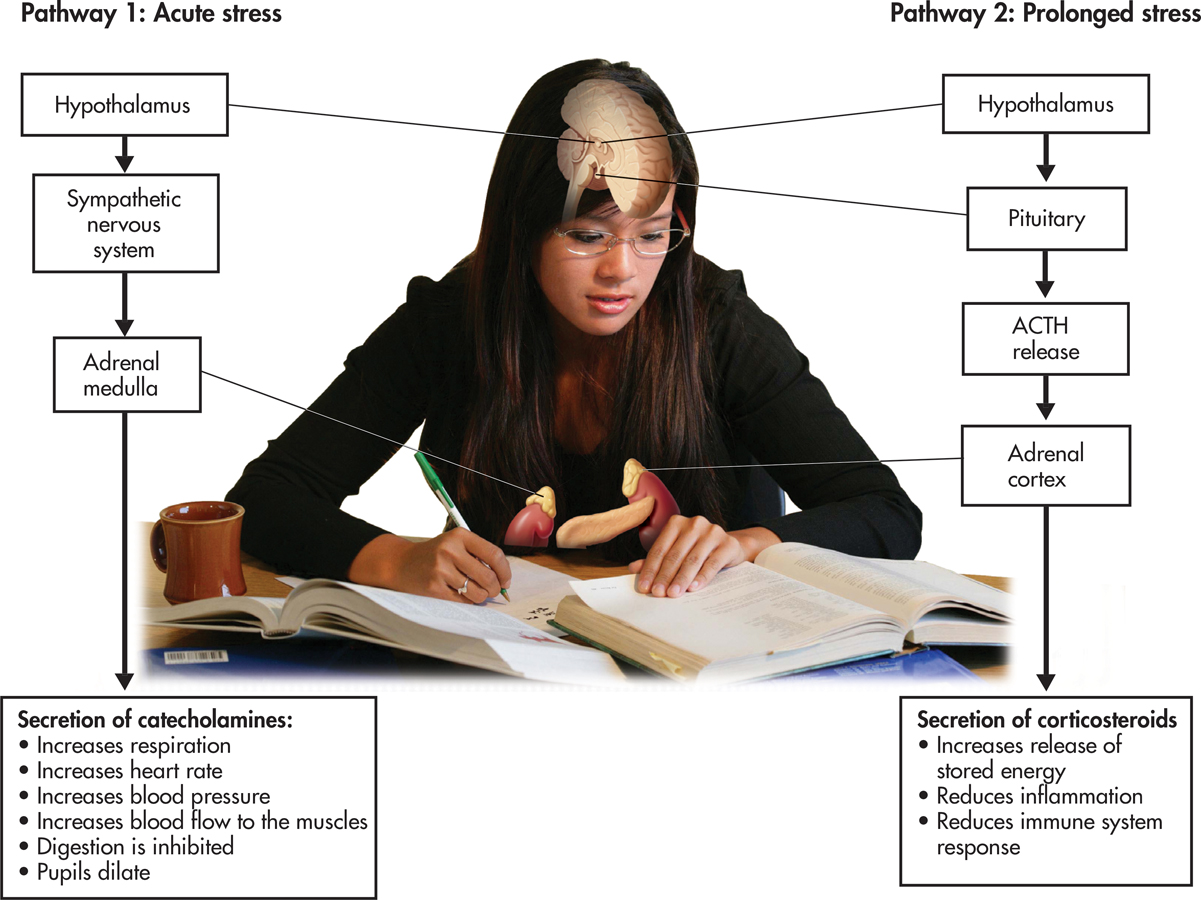
catecholamines
(cat-uh-COLE-uh-meenz) Hormones secreted by the adrenal medulla that cause rapid physiological arousal, including adrenaline and noradrenaline.
As a short-term reaction, the fight-or-flight response helps ensure survival by swiftly mobilizing internal physical resources to defensively attack or flee an immediate threat. Without question, the fight-or-flight response is very useful if you’re suddenly faced with a life-threatening situation, such as having to flee your house when you see a wall of flame come over the ridge. However, if exposure to a threat is prolonged, the intense arousal of the fight-or-flight response can also become prolonged. Under these conditions, Cannon believed, the fight-or-flight response could prove harmful to physical health.

HANS SELYE: STRESS AND THE GENERAL ADAPTATION SYNDROME
Cannon’s suggestion that prolonged stress could be physically harmful was confirmed by Canadian endocrinologist Hans Selye. Most of Selye’s pioneering research was done with rats that were exposed to prolonged stressors, such as electric shock, extreme heat or cold, or forced exercise. Regardless of the condition that Selye used to produce prolonged stress, he found the same pattern of physical changes in the rats. First, the adrenal glands became enlarged. Second, stomach ulcers and loss of weight occurred. And third, there was shrinkage of the thymus gland and lymph glands, two key components of the immune system. Selye believed that these distinct physical changes represented the essential effects of stress—the body’s response to any demand placed on it.
Selye (1956, 1976) found that prolonged stress activates a second endocrine pathway (see FIGURE 13.4) that involves the hypothalamus, the pituitary gland, and the adrenal cortex. In response to a stressor, the hypothalamus signals the pituitary gland to secrete a hormone called adrenocorticotropic hormone, abbreviated ACTH. In turn, ACTH stimulates the adrenal cortex to release stress-related hormones called corticosteroids, the most important of which is cortisol.
corticosteroids
(core-tick-oh-STER-oydz) Hormones released by the adrenal cortex that play a key role in the body’s response to long-term stressors.
In the short run, the corticosteroids provide several benefits, helping protect the body against the harm caused by stressors. For example, corticosteroids reduce inflammation of body tissues and enhance muscle tone in the heart and blood vessels. However, unlike the effects of catecholamines, which tend to diminish rather quickly, corticosteroids have long-lasting effects. If a stressor is prolonged, continued high levels of corticosteroids can weaken important body systems, lowering immunity and increasing susceptibility to physical symptoms of illness.
Selye (1976) showed that the devastating effects of prolonged stress developed in three progressive stages, producing what he termed the general adaptation syndrome. These stages include:
!launch!

Alarm stage: Catecholamines are released by the adrenal medulla. Intense arousal occurs, and the body mobilizes internal physical resources to meet the demands of the stress-producing event.
Resistance stage: As the body tries to adapt to the continuing stressful situation, physiological arousal lessens but remains above normal. Resistance to new stressors is impaired.
Exhaustion stage: If the stress-producing event persists, the symptoms of the alarm stage reappear, only now irreversibly. The body’s energy reserves become depleted and adaptation begins to break down, leading to exhaustion, physical disorders, and, potentially, death.
general adaptation syndrome
Hans Selye’s term for the three-stage progression of physical changes that occur when an organism is exposed to intense and prolonged stress. The three stages are alarm, resistance, and exhaustion.
Selye’s description of the general adaptation syndrome firmly established some of the critical biological links between stress-producing events and their potential impact on physical health. There is mounting evidence that chronic stress can lead to increased vulnerability to acute and chronic diseases, such as cardiovascular disease (G. E. Miller & others, 2009). Chronic stress has also been linked to psychological problems, such as major depressive disorder and anxiety (Hammen, 2005). And, as we’ll see in the next section, there is new evidence explaining the link between chronic stress and premature aging.
Question 13.3
UBEr7mFeSzukHiLvqa6wTKggR8rN6QJDvtFZOh0Lv3E3p3Ff4TCfvyq8lvTUnJ0lCn98XWz3mXf1St+Dr6bbvM9YV0Ki1XWvJ4hLG9apq3B8St2k2Z9z7Iwjv+k/vUHJ4aHSjhZL0STfxXIh6IoNXqn3S7ddyR7Jz9JVPC34EIKCJ/S2/bMgD8MDwZmDu3ejJmZs1Dny7FI8JV2Pi0WuxVHspMQDuTk/dtHup7aMEAEdlyeyA3y9yrUJm7u4MwF1JDwLuNM5U815BK7YStress, Chromosomes, and Aging: THE TELOMERE STORY
Diseases associated with aging and even premature aging itself have long been associated with chronic stress (Epel, 2009a; Kiecolt-Glaser & Glaser, 2010). But how might psychological stress affect physical aging?
One part of the answer may ultimately be found in the chromosomes. Telomeres are repeated, duplicate DNA sequences that are found at the very tips of chromosomes (see FIGURE 13.5). Like the plastic tips that protect shoelaces from fraying, telomeres protect the genetic data in the chromosomes from being broken or scrambled during cell division. With each cell division, the string of telomeres gets shorter. When telomeres become too short, the cell can no longer divide and may die or atrophy, causing tissue damage or loss. A growing body of literature has linked shorter telomeres with aging, age-related diseases, and mortality (Blackburn & Epel, 2012; Révész & others, 2014).
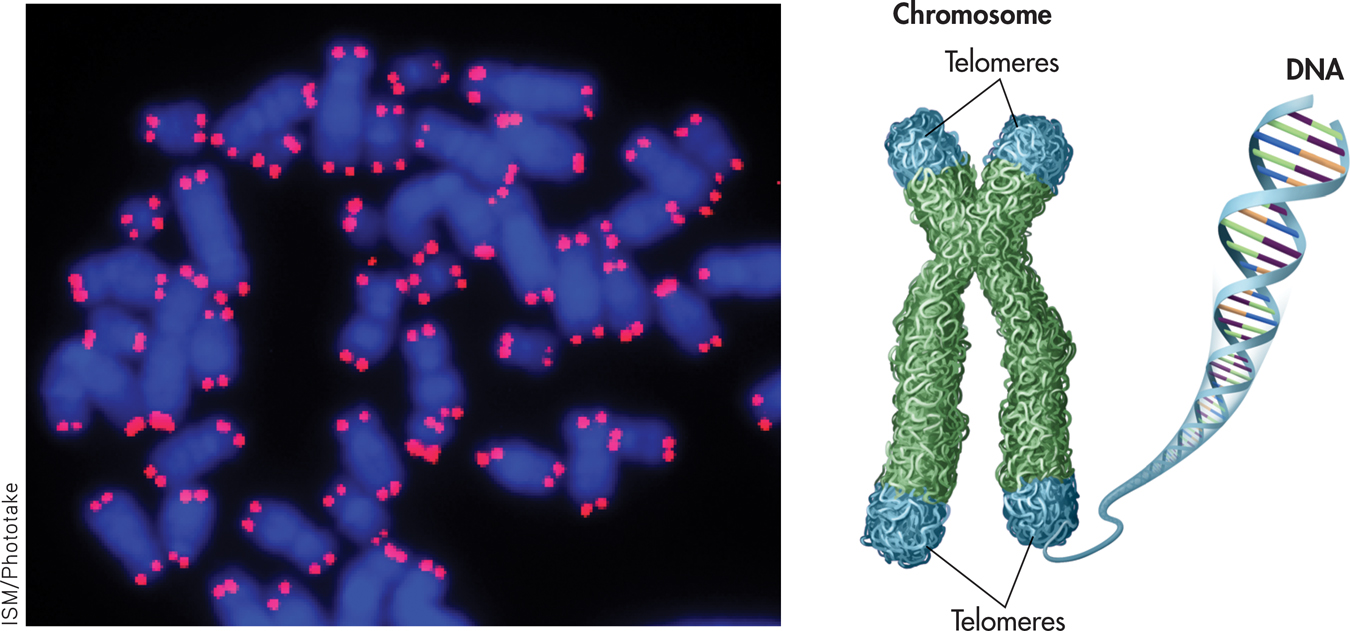
telomeres
Repeated, duplicate DNA sequences that are found at the very tips of chromosomes and that protect the chromosomes’ genetic data during cell division.
MYTH !rhtriangle! SCIENCE
Is it true that stress is linked to premature aging?
Although telomere length is roughly reflective of a cell’s age, the story is not that simple. Surprisingly, telomeres can also lengthen in response to physiological changes (Epel, 2009b). An enzyme called telomerase has the capacity to add DNA to shortened telomeres, rebuilding and extending the length of telomeres (Epel & others, 2010).
Could telomeres be implicated in the link between stress and premature aging? The tentative answer seems to be yes. First, several studies have linked elevated levels of stress hormones cortisol and the catecholamines to shorter telomeres (see Epel, 2009b). Second, researchers have discovered that people who are under chronic stress tend to have shortened telomeres.
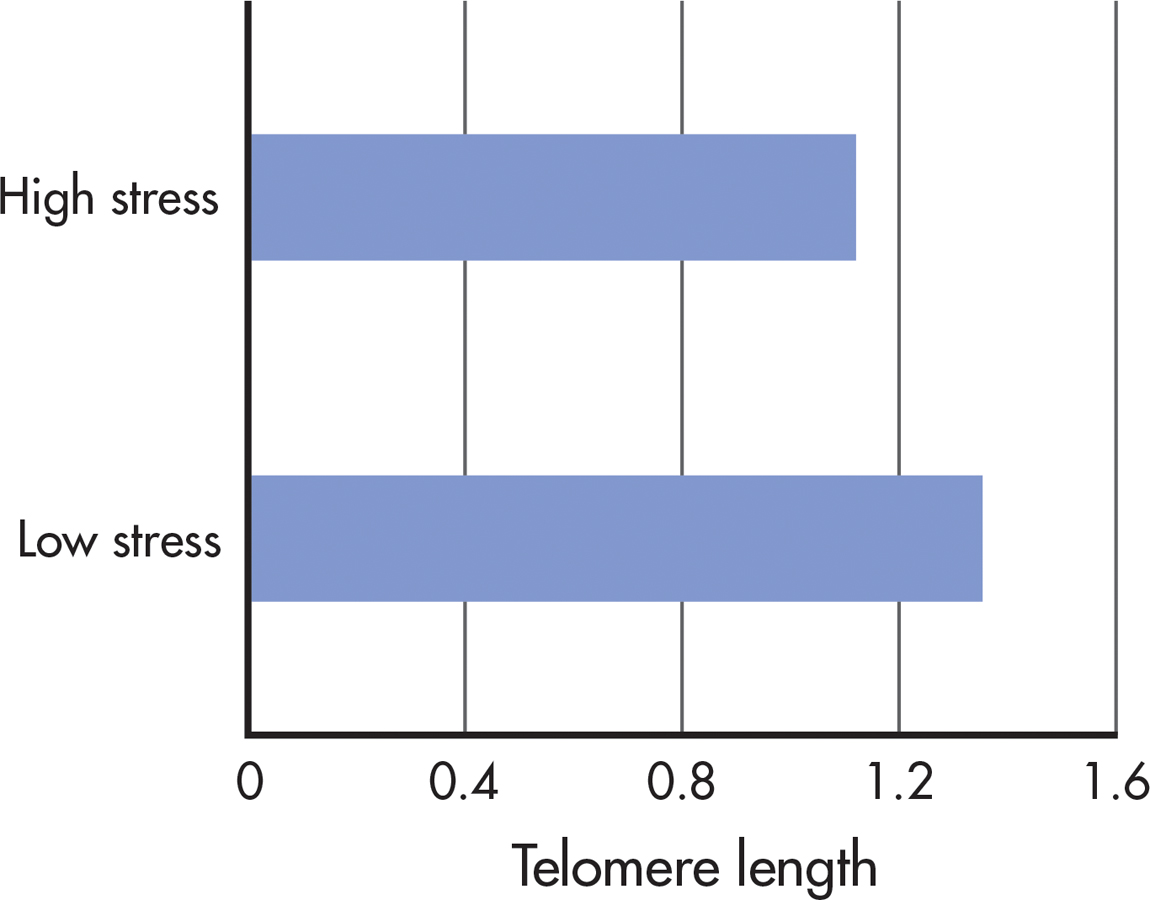
In a groundbreaking study that compared mothers of chronically ill children with mothers of healthy children, Elissa Epel and her colleagues (2004) found that caring for a chronically ill child was inversely associated with telomere length. That is, the longer the caregiving time, the shorter were the telomeres. Further, telomeres were shortest in mothers who perceived themselves as being under a great deal of stress, regardless of whether their children were healthy or ill (see FIGURE 13.6). Subsequent research has found that many types of chronic stress are associated with unusually short telomeres (Blackburn & Epel, 2012). For example, 9-year-old boys living in a stressful family environment had telomeres that were, on average, 40% shorter than 9-year-old boys living in nurturing family environments (Mitchell & others, 2014).
A clever study by Elissa Epel and colleagues (2010) provides the strongest evidence to date for a direct link between psychological stress and telomere length. Telomerase activity in “chronic-stress” older women who were caregivers for people with dementia was compared with telomerase activity in a “low-stress” matched control group. At the beginning of the study, telomerase activity was measured and found to be lower in the chronic-stress women than in the low-stress women.
All of the participants were exposed to an acute laboratory stressor: Each had to deliver an impromptu speech in front of stony-faced judges and then take a math test. How did telomerase respond to acute stress? Within 90 minutes of experiencing the acute stress, telomerase activity increased by almost 20% in both groups of women. And, those participants who perceived the laboratory stressor as most threatening had the highest telomerase response (see FIGURE 13.7).
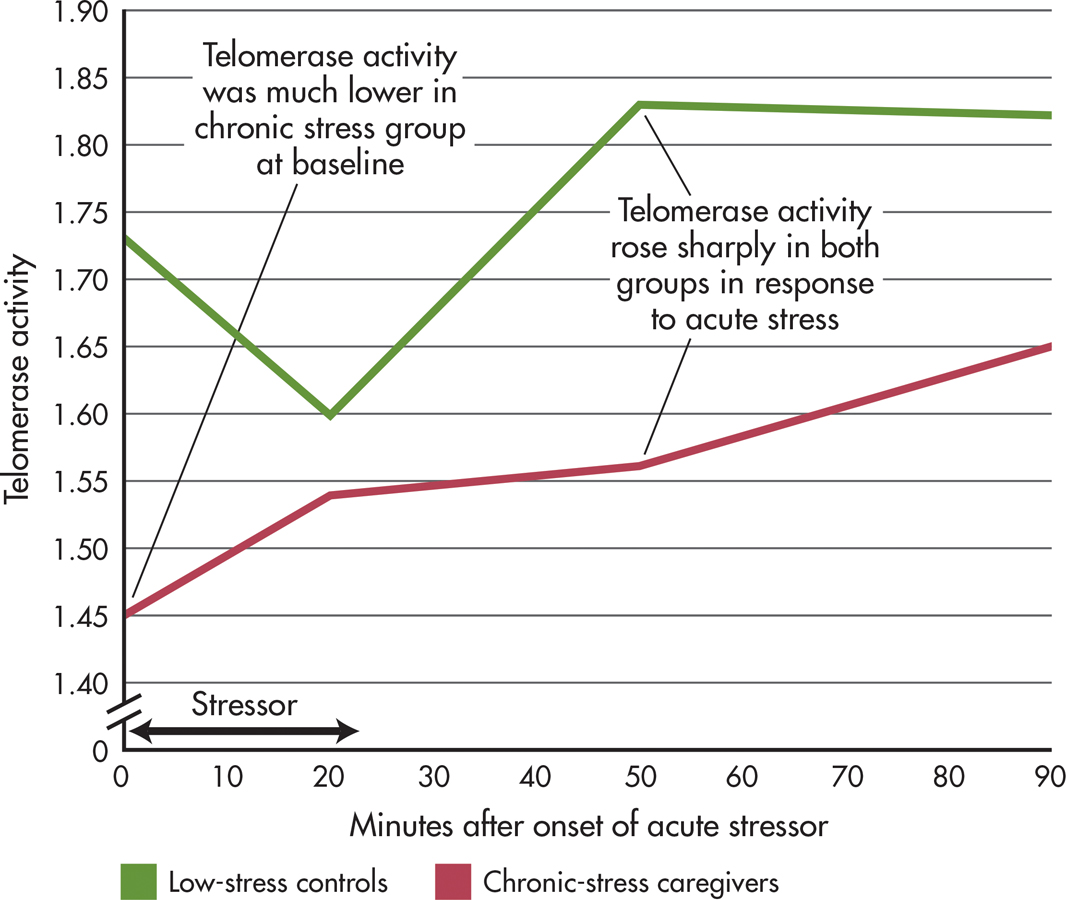
Epel’s research is the first experimental demonstration that telomerase activity is directly affected by acute psychological stress. Acute, short-term stress produced a positive, adaptive effect on telomerase activity; chronic stress had a negative, maladaptive effect on telomerase activity.
Although research on telomeres and telomerase activity is still in its infancy, new findings suggest potential interventions that might someday be used to target telomeres to improve health and prevent age-related degenerative diseases. For example, a recent study found that vigorous physical exercise helps protect telomeres from the effects of stress (Puterman & others, 2010). Researchers are also actively investigating the impact of factors like stress management, meditation, and diet on telomeres (Epel & others, 2009; Jacobs & others, 2011; Ornish & others, 2008).
Question 13.4
douBvPx7M8R3a5KV6wWoMMFCTpo5N/de6M7Q9VbryxkOJe+zdy5oipK1paVJQ4GCZwUMNSCzcWAyoPChcI7Aa02sSGgD410Lb+ZkZM2gzI8DjNGLEbznMqdJGoytukCuLBIXyNaNZPRA5wLyEZNamozYb4EnfHefuivr5v46kKVsh9T7etT5ilryuVWrKbxYuGp6KrPNqC7ii/s6U2a6xGHnBp6alT7NPwte3o65tTzmGkxVEMyLjg+bhMBt/Ct9VJlmch26PaE=Stress and the Immune System
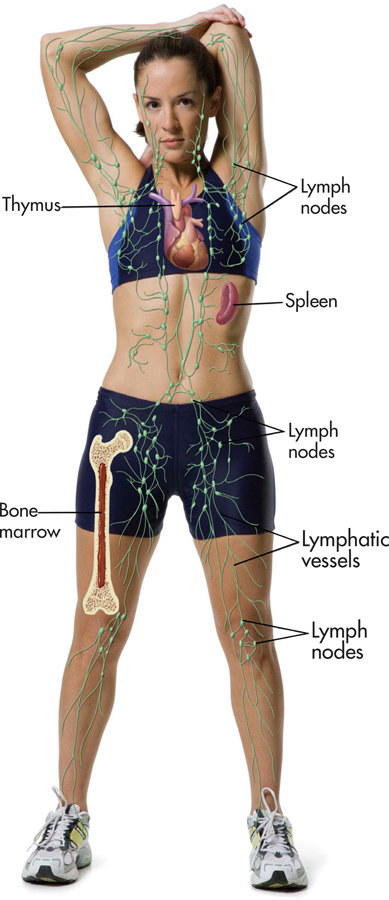
The immune system is your body’s surveillance system. It detects and battles foreign invaders, such as bacteria, viruses, and tumor cells. Your immune system comprises several organs, including bone marrow, the spleen, the thymus, and the lymph nodes (see FIGURE 13.8). The most important elements of the immune system are lymphocytes—the specialized white blood cells that fight bacteria, viruses, and other foreign invaders. Lymphocytes are initially manufactured in the bone marrow. From the bone marrow, they migrate to other immune system organs, such as the thymus and spleen, where they develop more fully and are stored until needed.
immune system
Body system that produces specialized white blood cells that protect the body from viruses, bacteria, and tumor cells.
lymphocytes
(LIMF-oh-sites) Specialized white blood cells that are responsible for immune defenses.
PSYCHONEUROIMMUNOLOGY
Until the 1970s, the immune system was thought to be completely independent of other body systems, including the nervous and endocrine systems. Thus, most scientists believed that psychological processes could not influence the immune system response.
However, research in a new interdisciplinary field called psychoneuroimmunology helped establish that there are many interconnections among these systems, including the brain (Ader, 1993). Psychoneuroimmunology is the scientific study of the connections among psychological processes (psycho-), the nervous system (-neuro-), and the immune system (-immunology).
psychoneuroimmunology
An interdisciplinary field that studies the interconnections among psychological processes, nervous and endocrine system functions, and the immune system.
First, the central nervous system and the immune system are directly linked via sympathetic nervous system fibers, which influence the production and functioning of lymphocytes.
Second, the surfaces of lymphocytes contain receptor sites for neurotransmitters and hormones, including catecholamines and cortisol. Thus, rather than operating independently, the activities of lymphocytes and the immune system are directly influenced by neurotransmitters, hormones, and other chemical messengers from the nervous and endocrine systems.
Third, psychoneuroimmunologists have discovered that lymphocytes themselves produce neurotransmitters and hormones. These neurotransmitters and hormones, in turn, influence the nervous and endocrine systems. In other words, there is ongoing interaction and communication among the nervous system, the endocrine system, and the immune system (Kendall-Tackett, 2010). Each system influences and is influenced by the other systems (Kiecolt-Glaser, 2009).
STRESSORS THAT CAN INFLUENCE THE IMMUNE SYSTEM
When researchers began studying how stress affects the immune system, they initially focused on extremely stressful events, such as the reentry of returning astronauts or being forced to stay awake for days (see Kiecolt-Glaser & Glaser, 1993). These highly stressful events, it turned out, were associated with reduced immune system functioning.
Subsequent research found that immune system functioning was also affected by more common stresses. For example, the stress caused by the end or disruption of important interpersonal relationships impairs immune function, putting people at greater risk for health problems (Kiecolt-Glaser & others, 2009). And perhaps not surprisingly, chronic stressors that continue for years, such as caring for a family member with Alzheimer’s disease, also diminish immune system functioning (Jeckel & others, 2010). Even ordinary stressors, such as marital arguments or the pressure of exams, can adversely affect the immune system (Lester & others, 2010).
What are the practical implications of reduced immune system functioning? One consistent finding is that psychological stress increases the length of time it takes for a wound to heal. In one study, dental students volunteered to receive two small puncture wounds on the roofs of their mouths (Marucha & others, 1998). To compare the impact of stress on wound healing, the students received the first wound when they were on summer vacation and the second wound three days before their first major exam during the fall term. The results? The wounds inflicted before the major test healed an average of 40 percent more slowly—an extra three days—than the wounds inflicted on the same volunteers during summer vacation. Other studies have shown similar findings (Glaser & Kiecolt-Glaser, 2005).
FOCUS ON NEUROSCIENCE
The Mysterious Placebo Effect
The placebo effect is perhaps one of the most dramatic examples of how the mind influences the body. A placebo is an inactive substance with no known effects, such as a sugar pill or an injection of sterile water. Placebos are often used in biomedical research to help gauge the effectiveness of an actual medication or treatment. But after being given a placebo, many research participants, including those suffering from pain or diseases, experience benefits from the placebo treatment. How can this be explained?
In Chapter 2, we noted that one possible way that placebos might reduce pain is by activating the brain’s own natural painkillers—the endorphins. (The endorphins are structurally similar to opioid painkillers, like morphine.) One reason for believing this is that a drug called naloxone, which blocks the brain’s endorphin response, also blocks the painkilling effects of placebos (Price & others, 2008). Might placebos reduce pain by activating the brain’s natural opioid network?
A brain imaging study by Swedish neuroscientist Predrag Petrovic and his colleagues (2002) tackled this question. In the study, painfully hot metal was placed on the back of each volunteer’s hand. Each volunteer was then given an injection of either an actual opioid painkiller or a saline solution placebo. About 30 seconds later, positron emission tomography (PET) was used to scan the participants’ brain activity.
Both the volunteers who received the painkilling drug and the volunteers who received the placebo treatment reported that the injection provided pain relief. In the two PET scans shown here, you can see that the genuine painkilling drug (left) and the placebo (right) activated the same brain area, called the anterior cingulate cortex (marked by the cross). The anterior cingulate cortex is known to contain many opioid receptors. Interestingly, the level of brain activity was directly correlated with the participants’ subjective perception of pain relief. The PET scan on the right shows the brain activity of those participants who had strong placebo responses.
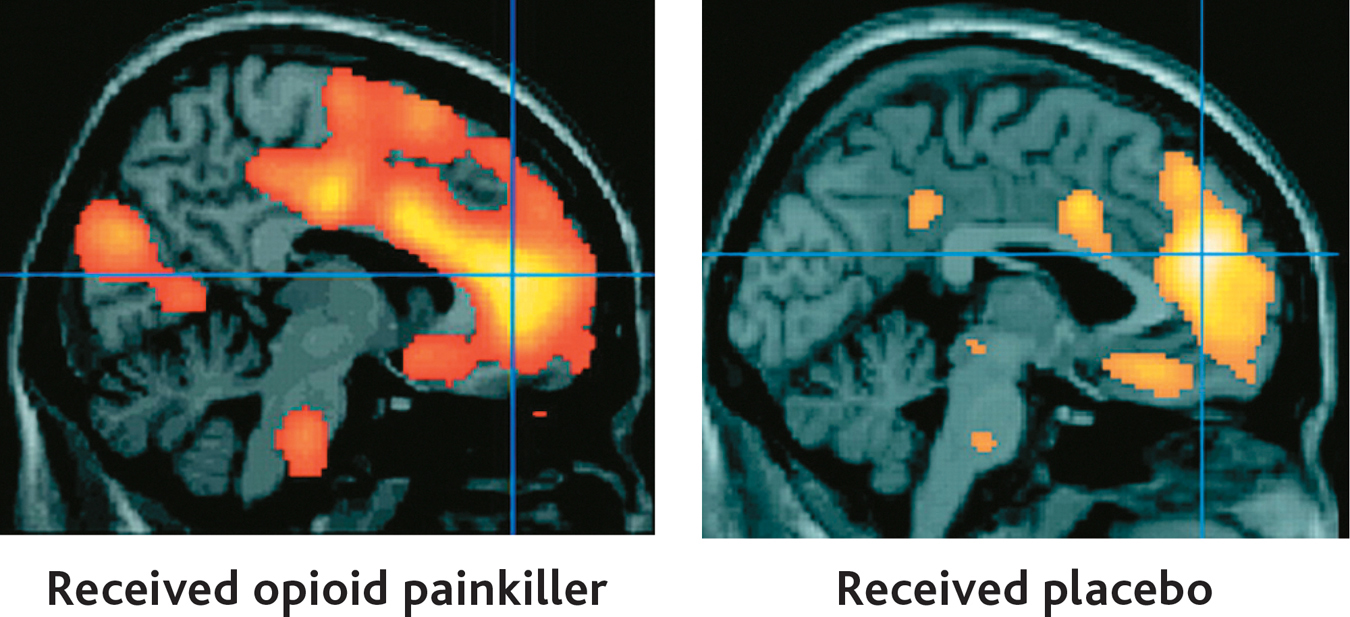
Many questions remain about exactly how placebos work, but studies by Petrovic (2010) and others have substantiated the biological reality of the placebo effect. For example, Jon-Kar Zubieta and his colleagues (2005) showed that a placebo treatment activated opioid receptors in several brain regions associated with pain. Further, the greater the activation, the higher the level of pain individual volunteers were able to tolerate. As these studies show, cognitive expectations, learned associations, and emotional responses can have a profound effect on the perception of pain. Research has also demonstrated that placebos produce measurable effects on other types of brain processes, including those of people experiencing Parkinson’s disease or major depressive disorder (de la Fuente-Fernández, 2009; Hunter & others, 2009).
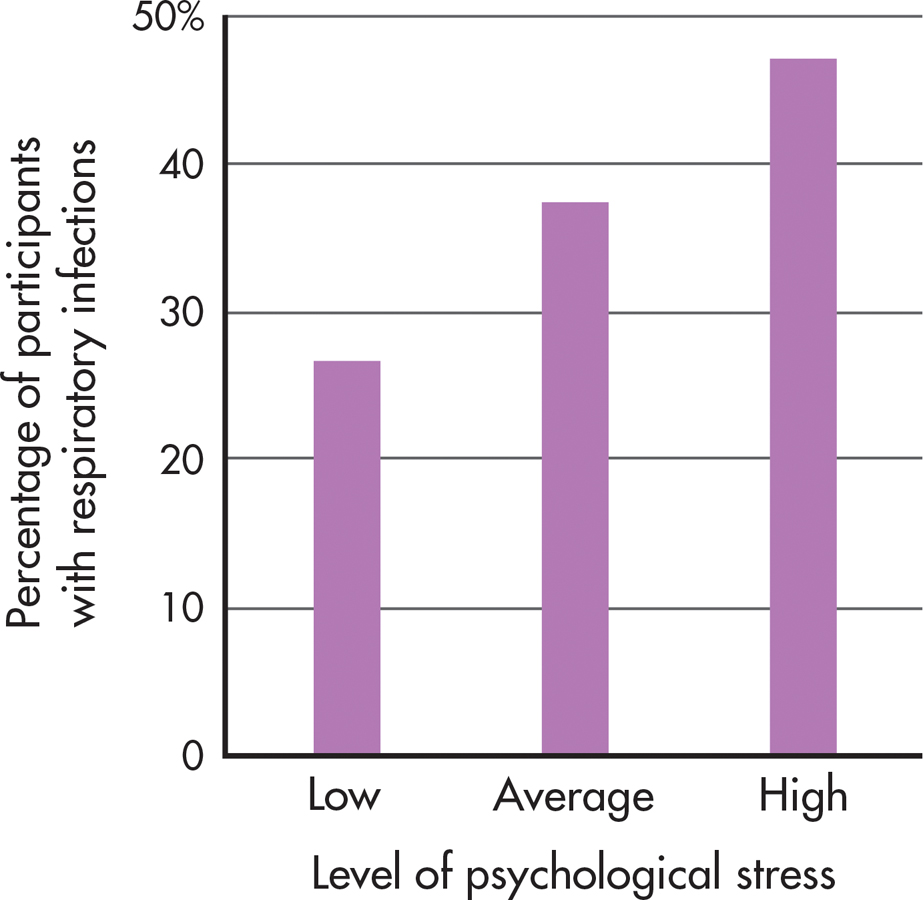
What about the relationship between stress and infection? In a series of carefully controlled studies, psychologist Sheldon Cohen and his colleagues (2006) demonstrated that people who are experiencing high levels of stress are more susceptible to infection by a cold virus than people who are not under stress (see FIGURE 13.9). Subjects who experienced chronic stressors that lasted a month or longer were most likely to develop colds after being exposed to a cold virus. One reason may be that chronic stress triggers the secretion of corticosteroids, which affect immune system functioning. Other research has shown that stress interferes with the effectiveness of influenza vaccinations (Pedersen & others, 2009).
Health psychologists have found that a wide variety of stressors are associated with diminished immune system functioning, increasing the risk of health problems and slowing recovery times (see Kiecolt-Glaser, 2009). However, while stress-related decreases in immune system functioning may heighten our susceptibility to health problems, exposure to stressors does not automatically translate into poorer health.
First, although prolonged, chronic stress impairs immune functioning, remember that acute, short-term stress actually enhances immune functioning (Dhabhar, 2011; Groer & others, 2010). Second, physical health is affected by the interaction of many factors, including heredity, nutrition, health-related habits, and access to medical care. Of course, your level of exposure to bacteria, viruses, and other sources of infection or disease will also influence your likelihood of becoming sick.

How do stressful events and negative emotions influence the immune system, and how big are the effects? This broad question has been intensely interesting to psychoneuroimmunology (PNI) researchers over the last three decades, and the consequent discoveries have substantially changed the face of health psychology.
—Janice Kiecolt-Glaser (2009)
Finally, the simple fact is that some people are more vulnerable to the negative effects of stress than others. Why? As you’ll see in the next section, researchers have found that a wide variety of psychological factors can influence people’s reactions to stressors (Pedersen & others, 2011).
Question 13.5
B90LFuhYecxeyICesb8SbhjQoHTxf1BohEiS3uGOSeHInolEnuhJx5B54ev8UF0fksHVb9zLQ3hxL2SnSmnZKcBNLePRtDSlml3MaOWLxypHwlwnzo1Wyd41y3vo8HHE7ZRCSwfspwg9hpn/tjxkH+NT/ERFNnxBvh3pXX3xObhCHiYY0mRUAJn0XzllJwx4yll0+urnfe3iMJjKpbMgmCWa9N/xCQTmhDMTjqi+659fi3dimdEUyvDmGZa+S4dSCONCEPT REVIEW 13.1
Sources and Effects of Stress
Indicate whether each of the following items is true or false.
1. According to the biopsychosocial model, physical well-being is primarily determined by genetic factors.
| A. | True |
| B. | False |
2. In the past year, Andrea has gotten divorced, changed jobs, moved three times, and lost her closest friend in an auto accident. Because of the number of stress-producing life events Andrea has experienced, she is certain to develop a major illness in the next six months.
| A. | True |
| B. | False |
3. The level of stress due to daily hassles is measured by the Social Readjustment Rating Scale.
| A. | True |
| B. | False |
4. During periods of prolonged stress, the adrenal medulla secretes stress-related hormones called catecholamines.
| A. | True |
| B. | False |
5. Shelley’s manager has just unexpectedly informed her that she is being laid off, effective immediately. Shelley is probably experiencing the exhaustion stage of the general adaptation syndrome.
| A. | True |
| B. | False |
6. The study of interconnections among the nervous system, the immune system, the endocrine system, and psychological factors is called psychoneuroimmunology.
| A. | True |
| B. | False |
Test your understanding of Introduction: Stress and Health Psychology and Physical Effects of Stress with
 .
.