2.4 Some Genes Discovered by Observing Segregation Ratios
Recall that one general aim of genetic analysis today is to dissect a biological property by discovering the set of single genes that affect it. We learned that an important way to identify these genes is by the phenotypic segregation ratios generated by their mutations–most often 1:1 and 3:1 ratios, both of which are based on equal segregation as defined by Gregor Mendel.
Let’s look at some examples that extend the Mendelian approach into a modern experimental setting. Typically, the researcher is confronted by an array of interesting mutant phenotypes that affect the property of interest (such as those depicted in Figure 2-1) and now needs to know whether they are inherited as single-mutant alleles. Mutant alleles can be either dominant or recessive, depending on their action; so the question of dominance also needs to be considered in the analysis.
The standard procedure is to cross the mutant with wild type. (If the mutant is sterile, then another approach is needed.) First, we will consider three simple cases that cover most of the possible outcomes:
A fertile flower mutant with no pigment in the petals (for example, white petaled in contrast with the normal red)
A fertile fruit-fly mutant with short wings
A fertile mold mutant that produces excess hyphal branches (hyperbranching)
A gene active in the development of flower color
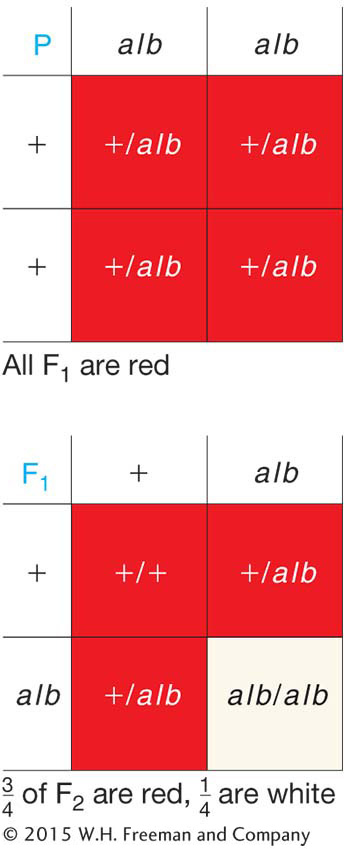
To begin the process, the white-flowered plant is crossed with the normal wild-type red. All the F1 plants are red flowered, and, of 500 F2 plants sampled, 378 are red flowered and 122 are white flowered. If we acknowledge the existence of sampling error, these F2 numbers are very close to a 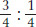 or 3:1, ratio. Because this ratio indicates single-gene inheritance, we can conclude that the mutant is caused by a recessive alteration in a single gene. According to the general rules of gene nomenclature, the mutant allele for white petals might be called alb for albino and the wild-type allele would be alb+ or just +. (The conventions for allele nomenclature vary somewhat among organisms: some of the variations are shown in Appendix A on nomenclature.) We surmise that the wild-type allele plays an essential role in producing the colored petals of the plant, a property that is almost certainly necessary for attracting pollinators to the flower. The gene might be implicated in the biochemical synthesis of the pigment or in the part of the signaling system that tells the cells of the flower to start making pigment or in a number of other possibilities that require further investigation. At the purely genetic level, the crosses made would be represented symbolically as
or 3:1, ratio. Because this ratio indicates single-gene inheritance, we can conclude that the mutant is caused by a recessive alteration in a single gene. According to the general rules of gene nomenclature, the mutant allele for white petals might be called alb for albino and the wild-type allele would be alb+ or just +. (The conventions for allele nomenclature vary somewhat among organisms: some of the variations are shown in Appendix A on nomenclature.) We surmise that the wild-type allele plays an essential role in producing the colored petals of the plant, a property that is almost certainly necessary for attracting pollinators to the flower. The gene might be implicated in the biochemical synthesis of the pigment or in the part of the signaling system that tells the cells of the flower to start making pigment or in a number of other possibilities that require further investigation. At the purely genetic level, the crosses made would be represented symbolically as
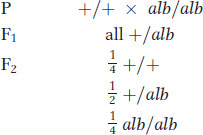
or graphically as in the grids on the right (see also Figure 2-5). This type of grid showing gametes and gametic fusions is called a Punnett square, named after an early geneticist, Reginald C. Punnett. They are useful devices for explaining genetic ratios. We shall encounter more in later discussions.
A gene for wing development
In the fruit-fly example, the cross of the mutant short-winged fly with wild-type long-winged stock yielded 788 progeny, classified as follows:
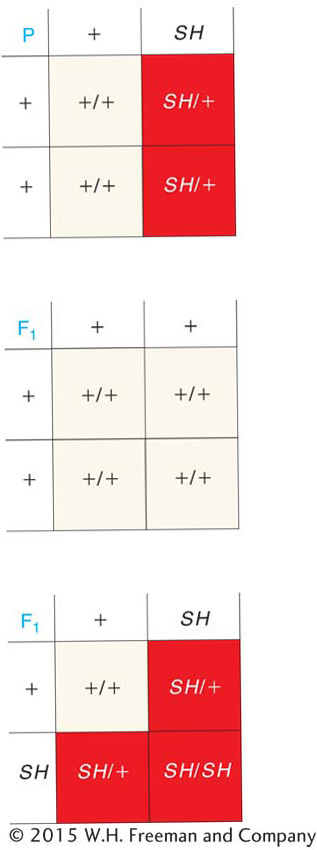
In total, there are 390 short- and 398 long-winged progeny, very close to a 1:1 ratio. The ratio is the same within males and females, again within the bounds of sampling error. Hence, from these results, the “short wings” mutant was very likely produced by a dominant mutation. Note that, for a dominant mutation to be expressed, only a single “dose” of mutant allele is necessary; so, in most cases, when the mutant first shows up in the population, it will be in the heterozygous state. (This is not true for a recessive mutation such as that in the preceding plant example, which must be homozygous to be expressed and must have come from the selfing of an unidentified heterozygous plant in the preceding generation.)
When long-winged progeny were interbred, all of their progeny were long winged, as expected of a recessive wild-type allele. When the short-winged progeny were interbred, their progeny showed a ratio of three-fourths short to one-fourth long.
Dominant mutations are represented by uppercase letters or words: in the present example, the mutant allele might be named SH, standing for “short.” Then the crosses would be represented symbolically as
or graphically as shown in the grids on the left.
This analysis of the fly mutant identifies a gene that is part of a subset of genes that, in wild-type form, are crucial for the normal development of a wing. Such a result is the starting point of further studies that would focus on the precise developmental and cellular ways in which the growth of the wing is arrested, which, once identified, reveal the time of action of the wild-type allele in the course of development.
A gene for hyphal branching
A hyperbranching fungal mutant (such as the button-like colony in Figure 2-1) was crossed with a wild-type fungus with normal sparse branching. In a sample of 300 progeny, 152 were wild type and 148 were hyperbranching, very close to a 1:1 ratio. We infer from this single-gene inheritance ratio that the hyperbranching mutation is of a single gene. In haploids, assigning dominance is usually not possible, but, for convenience, we can call the hyperbranching allele hb and the wild type hb+ or +. The cross must have been
The mutation and inheritance analysis has uncovered a gene whose wild-type allele is essential for normal control of branching, a key function in fungal dispersal and nutrient acquisition. Now the mutant needs to be investigated to see the location in the normal developmental sequence at which the mutant produces a block. This information will reveal the time and place in the cells at which the normal allele acts.
Sometimes, the severity of a mutant phenotype renders the organism sterile, unable to go through the sexual cycle. How can the single-gene inheritance of sterile mutants be demonstrated? In a diploid organism, a sterile recessive mutant can be propagated as a heterozygote and then the heterozygote can be selfed to produce the expected 25 percent homozygous recessive mutants for study. A sterile dominant mutant is a genetic dead end and cannot be propagated sexually, but, in plants and fungi, such a mutant can be easily propagated asexually.
What if a cross between a mutant and a wild type does not produce a 3:1 or a 1:1 ratio as discussed here, but some other ratio? Such a result can be due to the interactions of several genes or to an environmental effect. Some of these possibilities are discussed in Chapter 6.
Predicting progeny proportions or parental genotypes by applying the principles of single-gene inheritance
We can summarize the direction of analysis of gene discovery as follows:
Observe phenotypic ratios in progeny → Deduce genotypes of parents (A/A, A/a, or a/a)
However, the same principle of inheritance (essentially Mendel’s law of equal segregation) can also be used to predict phenotypic ratios in the progeny of parents of known genotypes. These parents would be from stocks maintained by the researcher. The types and proportions of the progeny of crosses such as A/A × A/a, A/A × a/a, A/a × A/a, and A/a × a/a can be easily predicted. In summary,
Cross parents of known genotypes → Predict phenotypic ratios in progeny
This type of analysis is used in general breeding to synthesize genotypes for research or for agriculture. It is also useful in predicting likelihoods of various outcomes in human matings in families with histories of single-gene diseases.
After single-gene inheritance has been established, an individual showing the dominant phenotype but of unknown genotype can be tested to see if the genotype is homozygous or heterozygous. Such a test can be performed by crossing the individual (of phenotype A/?) with a recessive tester strain a/a. If the individual is heterozygous, a 1:1 ratio will result (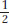 A/a and
A/a and 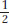 a/a); if the individual is homozygous, all progeny will show the dominant phenotype (all A/a). In general, the cross of an individual of unknown heterozygosity (for one gene or more) with a fully recessive parent is called a testcross, and the recessive individual is called a tester. We will encounter testcrosses many times throughout subsequent chapters; they are very useful in deducing the meiotic events taking place in more complex genotypes such as dihybrids and trihybrids. The use of a fully recessive tester means that meiosis in the tester parent can be ignored because all of its gametes are recessive and do not contribute to the phenotypes of the progeny. An alternative test for heterozygosity (useful if a recessive tester is not available and the organism can be selfed) is simply to self the unknown: if the organism being tested is heterozygous, a 3:1 ratio will be found in the progeny. Such tests are useful and common in routine genetic analysis.
a/a); if the individual is homozygous, all progeny will show the dominant phenotype (all A/a). In general, the cross of an individual of unknown heterozygosity (for one gene or more) with a fully recessive parent is called a testcross, and the recessive individual is called a tester. We will encounter testcrosses many times throughout subsequent chapters; they are very useful in deducing the meiotic events taking place in more complex genotypes such as dihybrids and trihybrids. The use of a fully recessive tester means that meiosis in the tester parent can be ignored because all of its gametes are recessive and do not contribute to the phenotypes of the progeny. An alternative test for heterozygosity (useful if a recessive tester is not available and the organism can be selfed) is simply to self the unknown: if the organism being tested is heterozygous, a 3:1 ratio will be found in the progeny. Such tests are useful and common in routine genetic analysis.
KEY CONCEPT
The principles of inheritance (such as the law of equal segregation) can be applied in two directions: (1) inferring genotypes from phenotypic ratios and (2) predicting phenotypic ratios from parents of known genotypes.

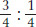 or 3:1, ratio. Because this ratio indicates single-
or 3:1, ratio. Because this ratio indicates single-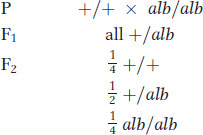
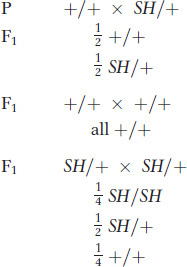
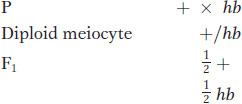
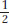 A/a and
A/a and 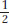 a/a); if the individual is homozygous, all progeny will show the dominant phenotype (all A/a). In general, the cross of an individual of unknown heterozygosity (for one gene or more) with a fully recessive parent is called a testcross, and the recessive individual is called a tester. We will encounter testcrosses many times throughout subsequent chapters; they are very useful in deducing the meiotic events taking place in more complex genotypes such as dihybrids and trihybrids. The use of a fully recessive tester means that meiosis in the tester parent can be ignored because all of its gametes are recessive and do not contribute to the phenotypes of the progeny. An alternative test for heterozygosity (useful if a recessive tester is not available and the organism can be selfed) is simply to self the unknown: if the organism being tested is heterozygous, a 3:1 ratio will be found in the progeny. Such tests are useful and common in routine genetic analysis.
a/a); if the individual is homozygous, all progeny will show the dominant phenotype (all A/a). In general, the cross of an individual of unknown heterozygosity (for one gene or more) with a fully recessive parent is called a testcross, and the recessive individual is called a tester. We will encounter testcrosses many times throughout subsequent chapters; they are very useful in deducing the meiotic events taking place in more complex genotypes such as dihybrids and trihybrids. The use of a fully recessive tester means that meiosis in the tester parent can be ignored because all of its gametes are recessive and do not contribute to the phenotypes of the progeny. An alternative test for heterozygosity (useful if a recessive tester is not available and the organism can be selfed) is simply to self the unknown: if the organism being tested is heterozygous, a 3:1 ratio will be found in the progeny. Such tests are useful and common in routine genetic analysis.