3.5 Organelle Genes: Inheritance Independent of the Nucleus
So far, we have considered how nuclear genes assort independently by virtue of their loci on different chromosomes. However, although the nucleus contains most of a eukaryotic organism’s genes, a distinct and specialized subset of the genome is found in the mitochondria, and, in plants, also in the chloroplasts. These subsets are inherited independently of the nuclear genome, and so they constitute a special case of independent inheritance, sometimes called extranuclear inheritance.
111
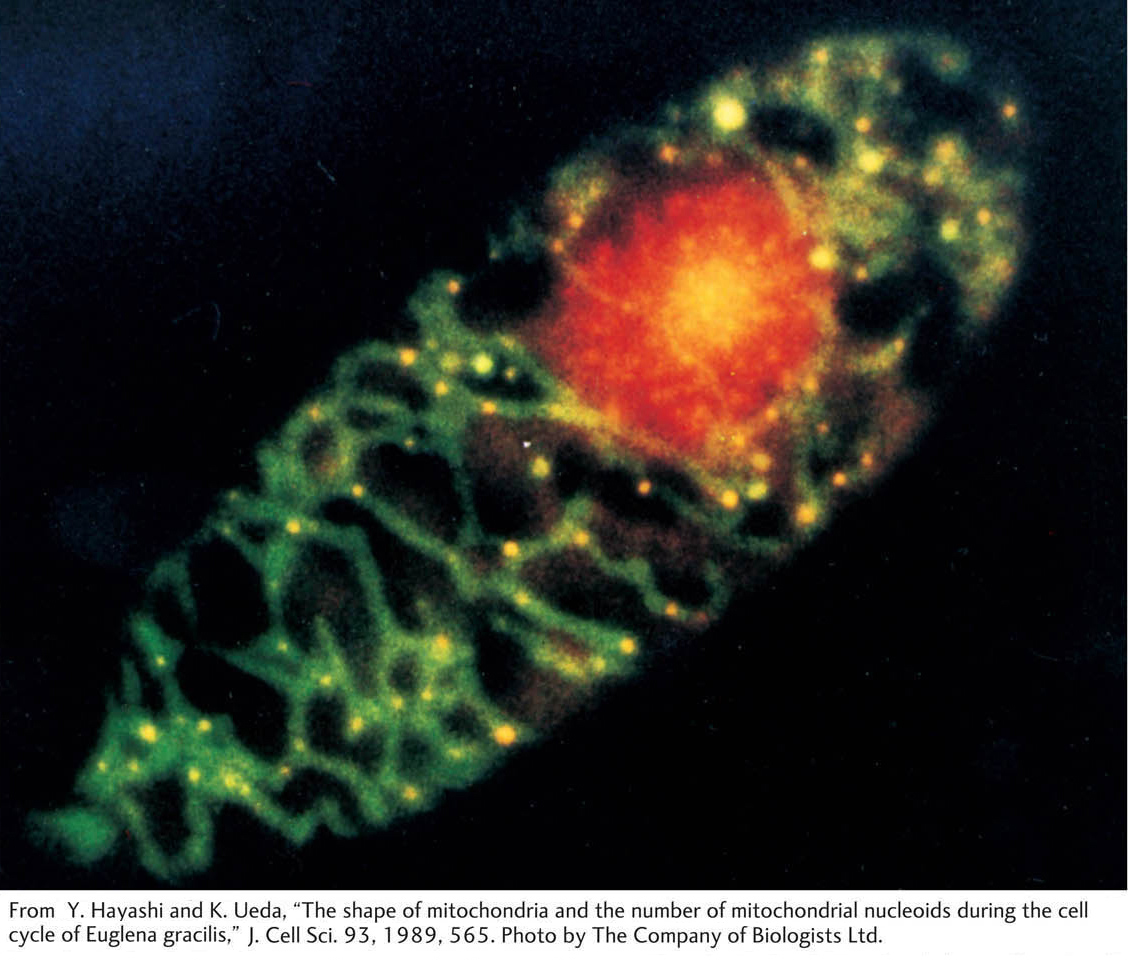
Mitochondria and chloroplasts are specialized organelles located in the cytoplasm. They contain small circular chromosomes that carry a defined subset of the total cell genome. Mitochondrial genes are concerned with the mitochondrion’s task of energy production, whereas chloroplast genes are needed for the chloroplast to carry out its function of photosynthesis. However, neither organelle is functionally autonomous because each relies to a large extent on nuclear genes for its function. Why some of the necessary genes are in the organelles themselves and others are in the nucleus is still something of a mystery, which will not be addressed here.
Another peculiarity of organelle genes is the large number of copies present in a cell. Each organelle is present in many copies per cell, and, furthermore, each organelle contains many copies of its chromosome. Hence, each cell can contain hundreds or thousands of organelle chromosomes. Consider chloroplasts, for example. Any green cell of a plant has many chloroplasts, and each chloroplast contains many identical circular DNA molecules, the so-
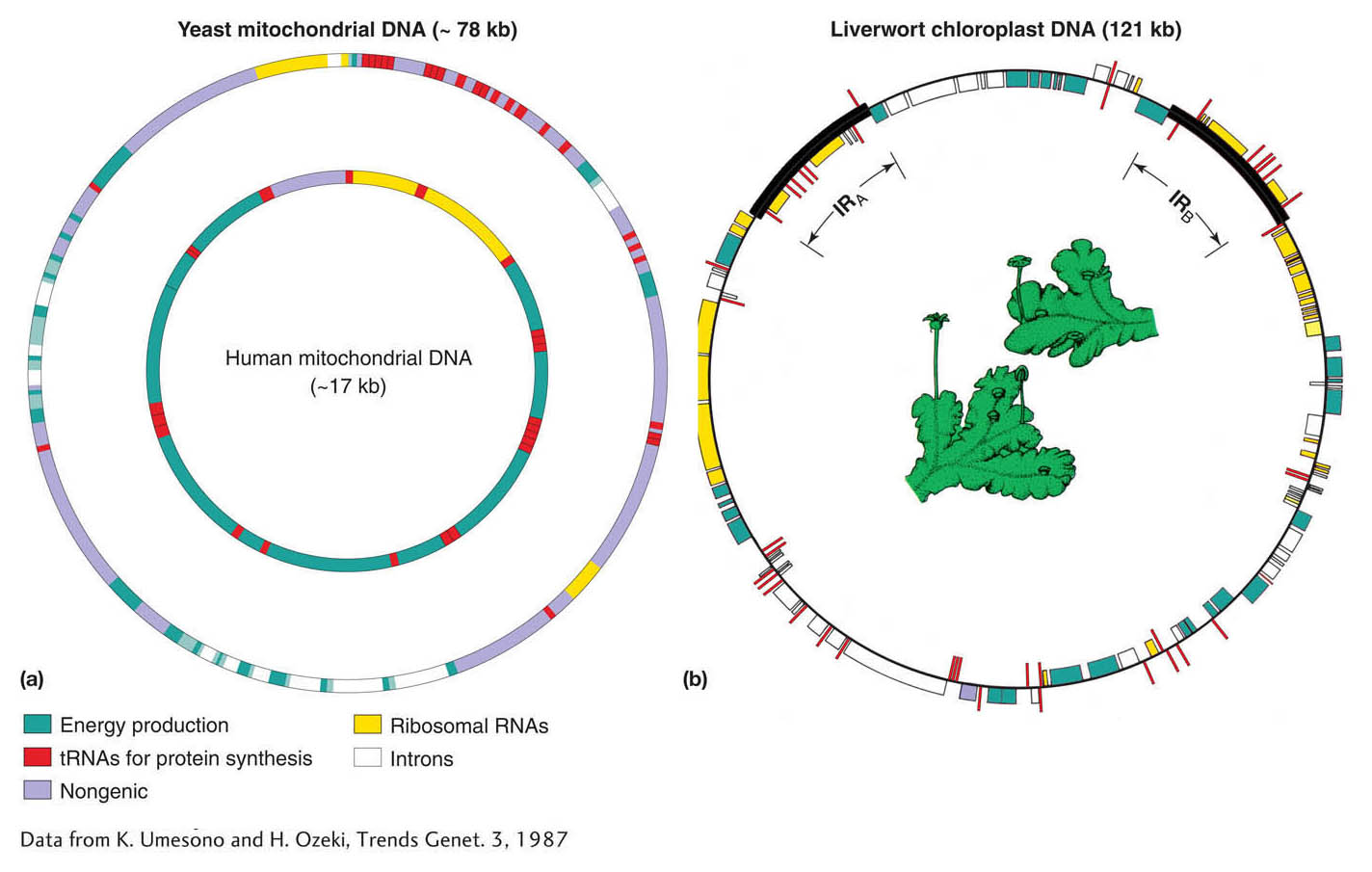
Many organelle chromosomes have now been sequenced. Examples of relative gene size and spacing in mitochondrial DNA (mtDNA) and chloroplast DNA (cpDNA) are shown in Figure 3-19. Organelle genes are very closely spaced, and, in some organisms, organelle genes can contain untranslated segments called introns. Note how most genes concern the chemical reactions taking place within the organelle itself: photosynthesis in chloroplasts and oxidative phosphorylation in mitochondria.
Patterns of inheritance in organelles
Organelle genes show their own special mode of inheritance called uniparental inheritance: progeny inherit organelle genes exclusively from one parent but not the other. In most cases, that parent is the mother, a pattern called maternal inheritance. Why only the mother? The answer lies in the fact that the organelle chromosomes are located in the cytoplasm and the male and female gametes do not contribute cytoplasm equally to the zygote. In regard to nuclear genes, both parents contribute equally to the zygote. However, the egg contributes the bulk of the cytoplasm, whereas the sperm contributes essentially none. Therefore, because organelles reside in the cytoplasm, the female parent contributes the organelles along with the cytoplasm, and essentially none of the organelle DNA in the zygote is from the male parent.
112
Some phenotypic variants are caused by a mutant allele of an organelle gene, and we can use these mutants to track patterns of organelle inheritance. We will temporarily assume that the mutant allele is present in all copies of the organelle chromosome, a situation that is indeed often found. In a cross, the variant phenotype will be transmitted to progeny if the variant used is the female parent, but not if it is the male parent. Hence, generally, cytoplasmic inheritance shows the following pattern:
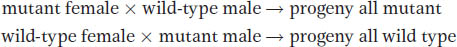
Indeed, this inheritance pattern is diagnostic of organelle inheritance in cases in which the genomic location of a mutant allele is not known.
Maternal inheritance can be clearly demonstrated in certain mutants of fungi. For example, in the fungus Neurospora, a mutant called poky has a slow-
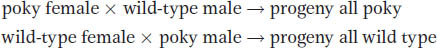
Sequencing has shown that the poky phenotype is caused by a mutation of a ribosomal RNA gene in mtDNA. Its inheritance is shown diagrammatically in Figure 3-20. The cross includes an allelic difference (ad and ad+) in a nuclear gene in addition to poky; notice how the Mendelian inheritance of the nuclear gene is independent of the maternal inheritance of the poky phenotype.
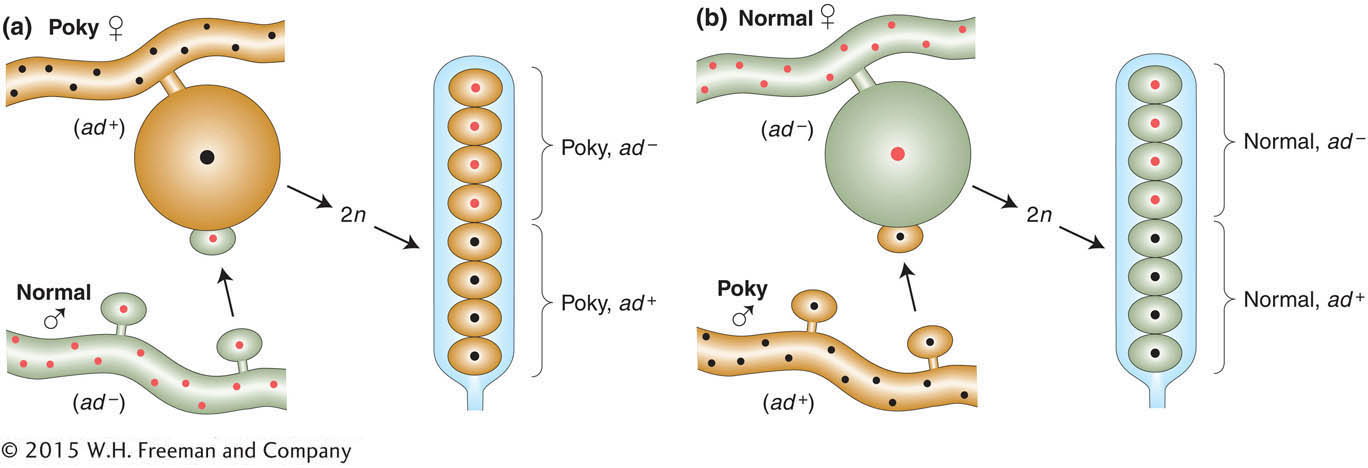
113
KEY CONCEPT
Variant phenotypes caused by mutations in cytoplasmic organelle DNA are generally inherited maternally and independent of the Mendelian patterns shown by nuclear genes.Cytoplasmic segregation
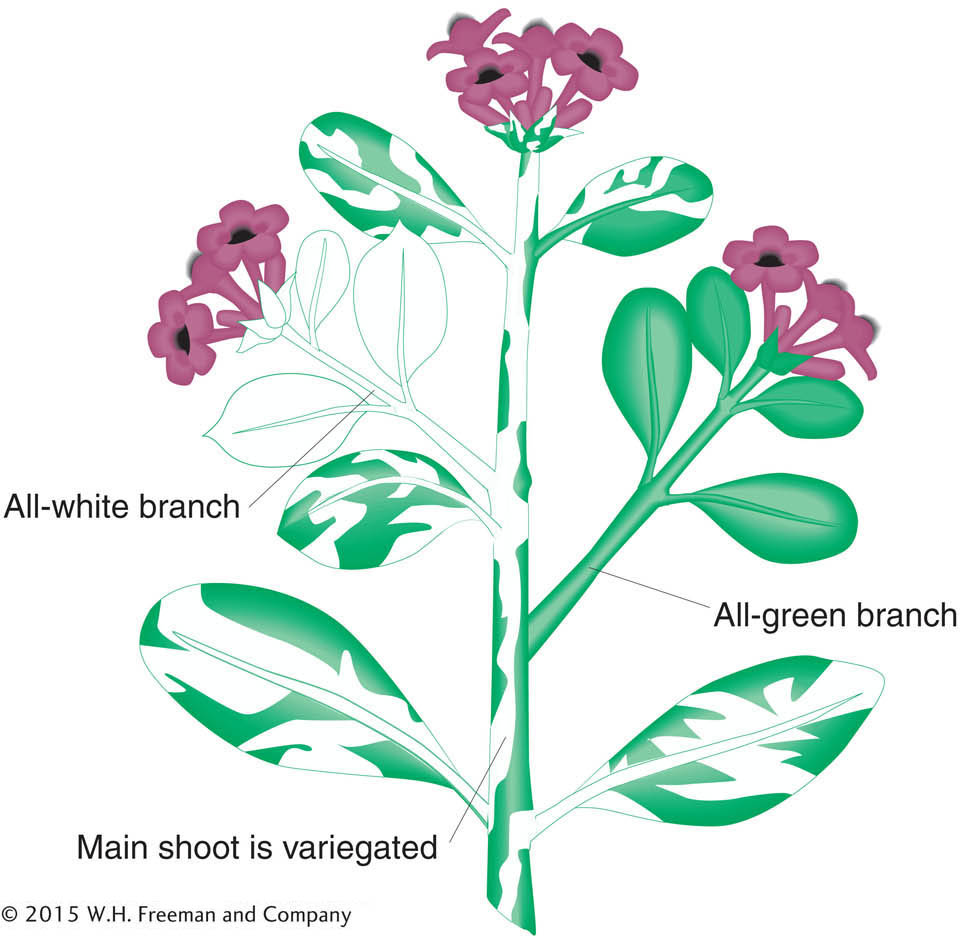
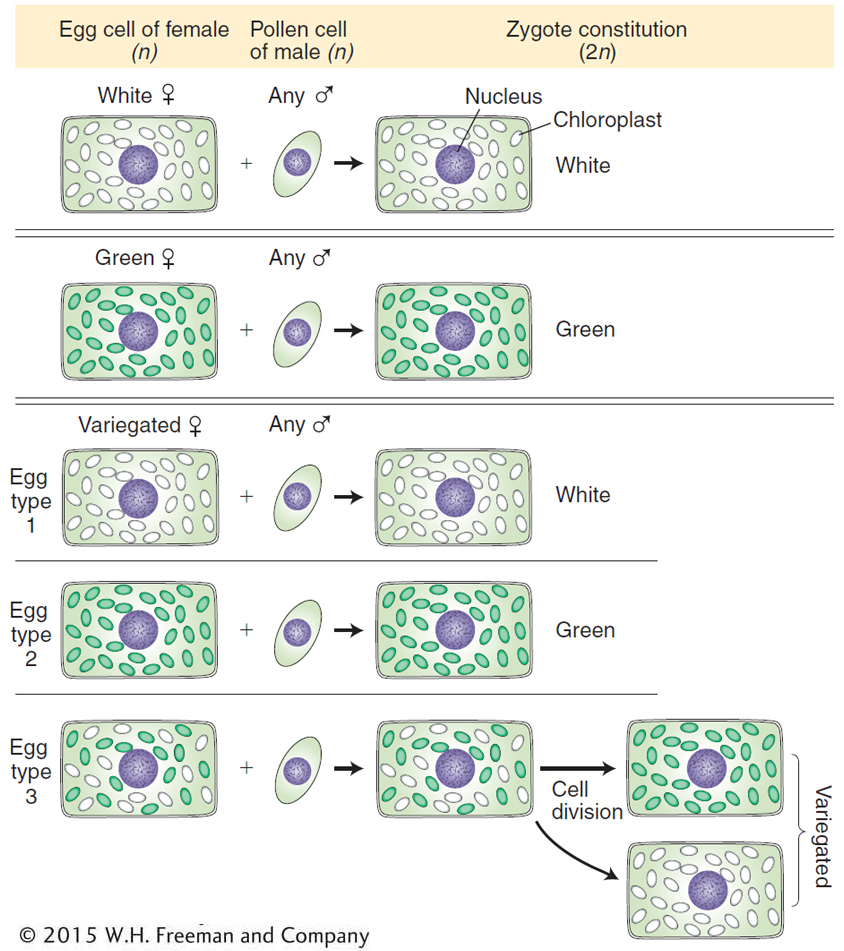
In some cases, cells contain mixtures of mutant and normal organelles. These cells are called cytohets, or heteroplasmons. In these mixtures, a type of cytoplasmic segregation can be detected, in which the two types apportion themselves into different daughter cells. The process most likely stems from chance partitioning of the multiple organelles in the course of cell division. Plants provide a good example. Many cases of white leaves are caused by mutations in chloroplast genes that control the production and deposition of the green pigment chlorophyll. Because chlorophyll is necessary for a plant to live, this type of mutation is lethal, and white-
The four-
114
The variegated zygotes (bottom of Figure 3-22) demonstrate cytoplasmic segregation. These variegated progeny come from eggs that are cytohets. Interestingly, when such a zygote divides, the white and green chloroplasts often segregate; that is, they sort themselves into separate cells, yielding the distinct green and white sectors that cause the variegation in the branches. Here, then, is a direct demonstration of cytoplasmic segregation.
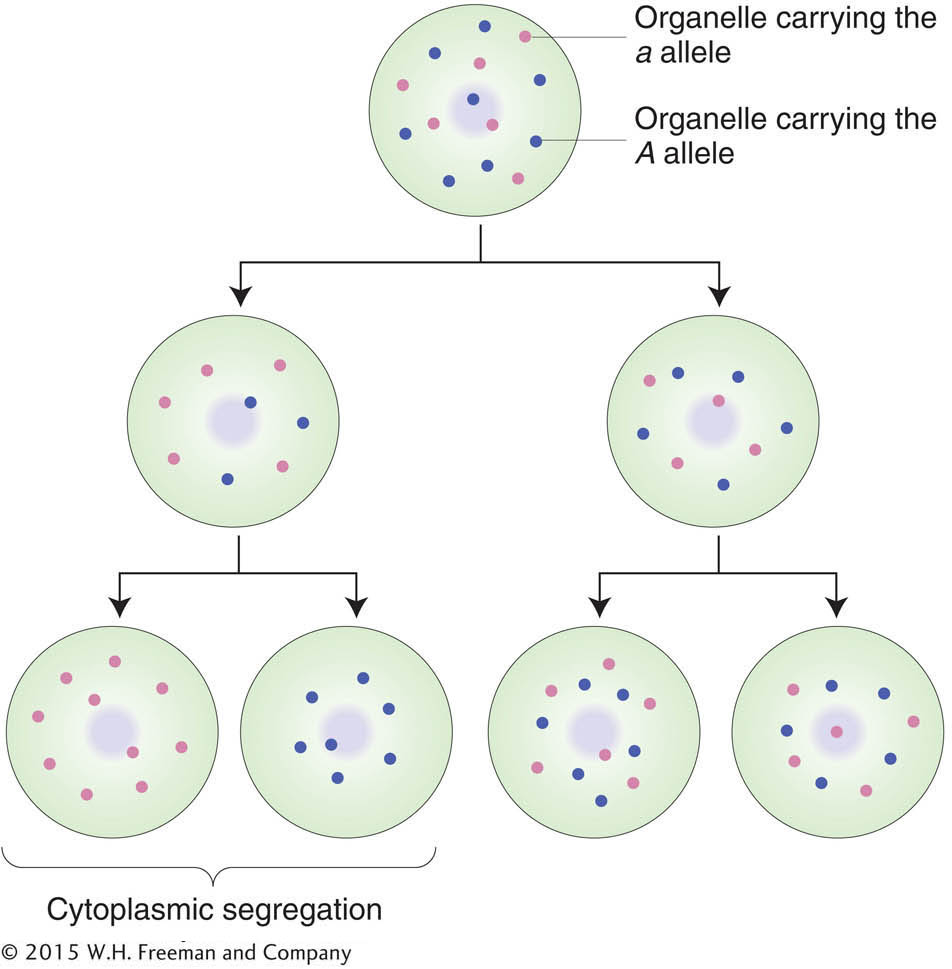
Given that a cell is a population of organelle molecules, how is it ever possible to obtain a “pure” mutant cell, containing only mutant chromosomes? Most likely, pure mutants are created in asexual cells as follows. The variants arise by mutation of a single gene in a single chromosome. Then, in some cases, the mutation-
115
KEY CONCEPT
Organelle populations that contain mixtures of two genetically distinct chromosomes often show segregation of the two types into the daughter cells at cell division. This process is called cytoplasmic segregation.In certain special systems such as in fungi and algae, cytohets that are “dihybrid” have been obtained (say, AB in one organelle chromosome and ab in another). In such cases, rare crossover-
KEY CONCEPT
Alleles on organelle chromosomes- in sexual crosses are inherited from one parent only (generally the maternal parent) and hence show no segregation ratios of the type nuclear genes do.
- in asexual cells can show cytoplasmic segregation.
- in asexual cells can occasionally show processes analogous to crossing over.
Cytoplasmic mutations in humans
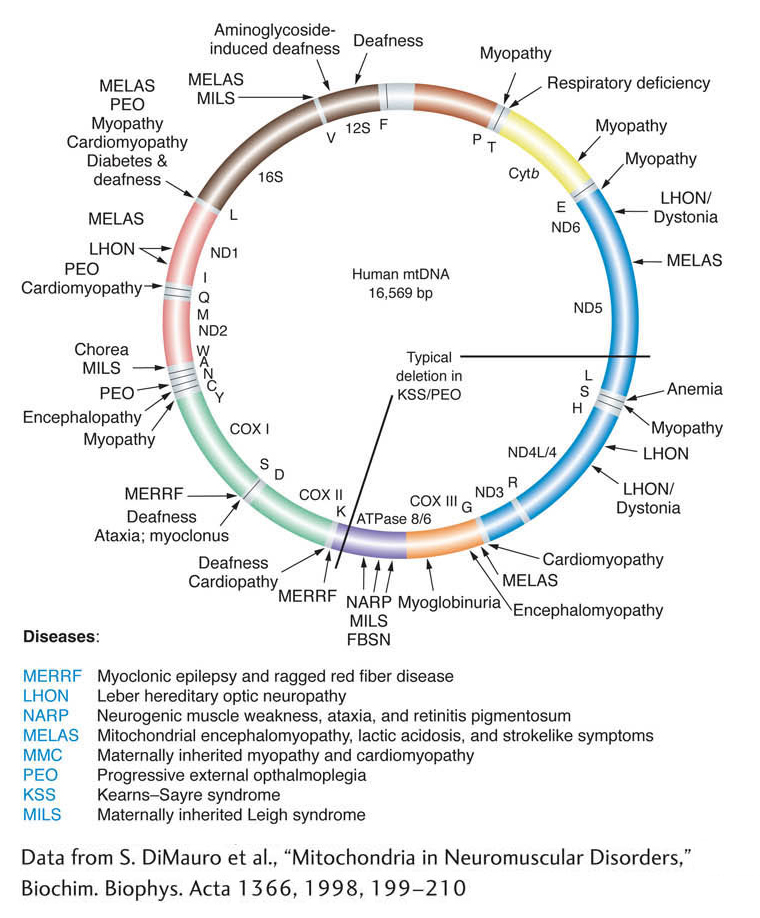
Are there cytoplasmic mutations in humans? Some human pedigrees show the transmission of rare disorders only through females and never through males. This pattern strongly suggests cytoplasmic inheritance and points to a mutation in mtDNA as the reason for the phenotype. The disease MERRF (myoclonic epilepsy and ragged red fiber) is such a phenotype, resulting from a single base change in mtDNA. It is a disease that affects muscles, but the symptoms also include eye and hearing disorders. Another example is Kearns-
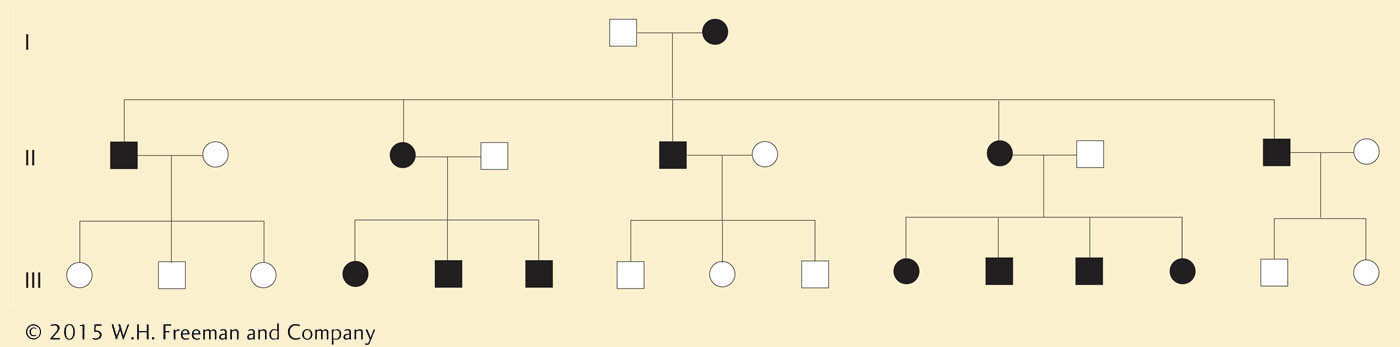
Figure 3-24 shows some of the mutations in human mitochondrial genes that can lead to disease when, by random drift and cytoplasmic segregation, they rise in frequency to such an extent that cell function is impaired. The inheritance of a human mitochondrial disease is shown in Figure 3-25. Note that the condition is always passed to offspring by mothers and never fathers. Occasionally, a mother will produce an unaffected child (not shown), probably owing to cyto-
116
mtDNA in evolutionary studies
Differences and similarities of homologous mtDNA sequences between species have been used extensively to construct evolutionary trees. Furthermore, it has been possible to introduce some extinct organisms into evolutionary trees using mtDNA sequences obtained from the remains of extinct organisms, such as skins and bones in museums. mtDNA evolves relatively rapidly, so this approach has been most useful in plotting recent evolution such as the evolution of humans and other primates. One key finding is that the “root” of the human mtDNA tree is in Africa, suggesting that Homo sapiens originated in Africa and from there dispersed throughout the world (see Chapter 18).
117