4.8 The Molecular Mechanism of Crossing Over
In this chapter we have analyzed the genetic consequences of the cytologically visible process of crossing over without worrying about the mechanism of crossing over. However, crossing over is remarkable in itself as a molecular process: how can two large coiled molecules of DNA exchange segments with a precision so exact that no nucleotides are lost or gained?
Studies on fungal octads gave a clue. Although most octads show the expected 4:4 segregation of alleles such as 4A : 4a, some rare octads show aberrant ratios. There are several types, but as an example we will use 5:3 octads (either 5A : 3a or 5a : 3A). Two things are peculiar about this ratio. First, there is one too many spores of one allele and one too few of the other. Second, there is a nonidentical sister-spore pair. Normally, postmeiotic replication gives identical sister-spore pairs as follows: the A A a a tetrad becomes
(the hyphens show sister spores). In contrast, an aberrant 5A : 3a octad must be
In other words, there is one nonidentical sister-spore pair (in bold).
The observation of a nonidentical sister-spore pair suggests that the DNA of one of the final four meiotic homologs contains heteroduplex DNA. Heteroduplex DNA is DNA in which there is a mismatched nucleotide pair in the gene under study. The logic is as follows. If in a cross of A × a, one allele (A) is G : C and the other allele (a) is A : T, the two alleles would usually replicate faithfully. However, a heteroduplex, which forms only rarely, would be a mismatched nucleotide pair such G : T or A : C (effectively a DNA molecule bearing both A and a information). Note that a heteroduplex involves only one nucleotide position: the surrounding DNA segment might be as follows, where the heteroduplex site is shown in bold:
At replication to form an octad, a G : T heteroduplex would pull apart and replicate faithfully, with G bonding to C and A bonding to T. The result would be a nonidentical spore pair of G : C (allele A) and A : T (allele a).
Nonidentical sister spores (and aberrant octads generally) were found to be statistically correlated with crossing over in the region of the gene concerned, providing an important clue that crossing over might be based on the formation of heteroduplex DNA.
In the currently accepted model (follow it in Figure 4-21), the heteroduplex DNA and a crossover are both produced by a double-stranded break in the DNA of one of the chromatids participating in the crossover. Let’s see how that works. Molecular studies show that broken ends of DNA will promote recombination between different chromatids. In step 1, both strands of a chromatid break in the same location. From the break, DNA is eroded at the 5′ end of each broken strand, leaving both 3′ ends single stranded (step 2). One of the single strands “invades” the DNA of the other participating chromatid; that is, it enters the center of the helix and base-pairs with its homologous sequence (step 3), displacing the other strand. Then the tip of the invading strand uses the adjacent sequence as a template for new polymerization, which proceeds by forcing the two resident strands of the helix apart (step 4). The displaced single-stranded loop hydrogen bonds with the other single strand (the blue one in the figure). If the invasion and strand displacement spans a site of heterozygosity (such as A/a), then a region of heteroduplex DNA is formed. Replication also takes place from the other single-stranded end to fill the gap left by the invading strand (also shown on the upper blue strand in step 4 of Figure 4-21). The replicated ends are sealed, and the net result is a strange structure with two single-stranded junctions called Holliday junctions after their original proposer, Robin Holliday. These junctions are potential sites of single-strand breakage and reunion; two such events, shown by the darts in the figure, then lead to a complete double-stranded crossover (step 5).
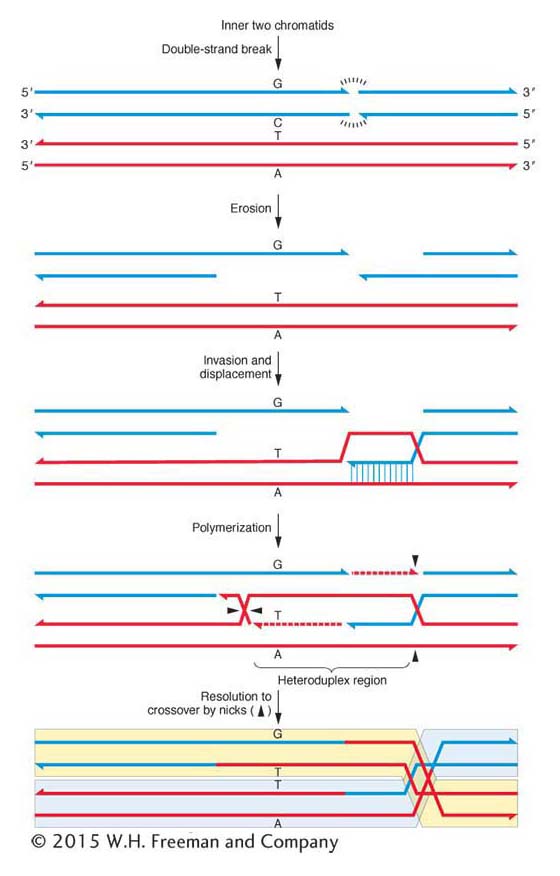
Figure 4-21: Crossing over creates heteroduplex DNA
Figure 4-21: A molecular model of crossing over. Only the two chromatids (blue and red) participating in the crossover are shown. The 3′-to-5′ strand is placed on the inside of both for clarity. The chromatids differ at one site, GC, in one allele (perhaps allele A) and AT in the other (perhaps a). Only the outcome with mispaired heteroduplex DNA and a crossover are shown. The final crossover products are shaded in yellow and blue.
Note that when the invading strand uses the invaded DNA as a replication template, this automatically results in an extra copy of the invaded sequence at the expense of the invading sequence, thus explaining the departure from the expected 4:4 ratio.
This same sort of recombination takes place at many different chromosomal sites where the invasion and strand displacement do not span a heterozygous mutant site. Here DNA would be formed that is heteroduplex in the sense that it is composed of strands of each participating chromatid, but there would not be a mismatched nucleotide pair and the resulting octad would contain only identical spore pairs. Those rare occasions in which the invasion and polymerization do span a heterozygous site are simply lucky cases that provided the clue for the mechanism of crossing over.
KEY CONCEPT
A crossover is initiated by a double-stranded break in the DNA of a chromatid at meiosis. A series of molecular events ensues that eventually produces crossover DNA molecules. (In addition, if the site of the crossover happens to be near a site of DNA heterozygosity in meiosis, aberrant non-Mendelian allele ratios for the heterozygous site may be produced.
