5.2 Bacterial conjugation
The earliest studies in bacterial genetics revealed the unexpected process of cell conjugation.
Discovery of conjugation
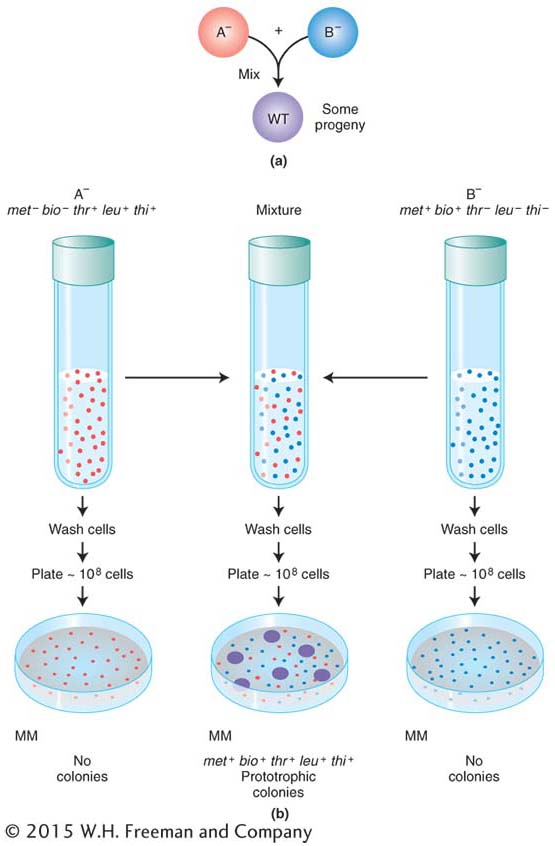
Do bacteria possess any processes similar to sexual reproduction and recombination? The question was answered by the elegantly simple experimental work of Joshua Lederberg and Edward Tatum, who in 1946 discovered a sex-
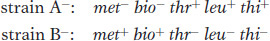
Figure 5-5a displays in simplified form the design of their experiment. Strains A− and B− were mixed together, incubated for a while, and then plated on minimal medium, on which neither auxotroph could grow. A small minority of the cells (1 in 107) was found to grow as prototrophs and, hence, must have been wild type, having regained the ability to grow without added nutrients. Some of the dishes were plated only with strain A− bacteria and some only with strain B− bacteria to act as controls, but no prototrophs arose from these platings. Figure 5-5b illustrates the experiment in more detail. These results suggested that some form of recombination of genes had taken place between the genomes of the two strains to produce the prototrophs.
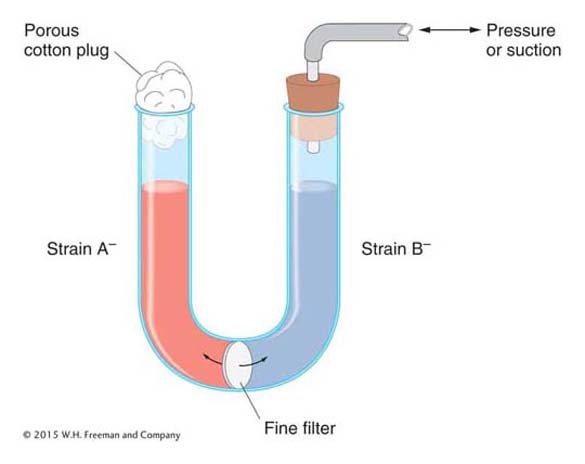
It could be argued that the cells of the two strains do not really exchange genes but instead leak substances that the other cells can absorb and use for growing. This possibility of “cross-
178
Discovery of the fertility factor (F)
In 1953, William Hayes discovered that, in the types of “crosses” just described here, the conjugating parents acted unequally (later, we will see ways to demonstrate this unequal participation). One parent (and only that parent) seemed to transfer some or all of its genome into another cell. Hence, one cell acts as a donor, and the other cell acts as a recipient. This “cross” is quite different from eukaryotic crosses in which parents contribute nuclear genomes equally to a progeny individual.
KEY CONCEPT
The transfer of genetic material in E. coli conjugation is not reciprocal. One cell, the donor, transfers part of its genome to the other cell, which acts as the recipient.179
By accident, Hayes discovered a variant of his original donor strain that would not produce recombinants on crossing with the recipient strain. Apparently, the donor-
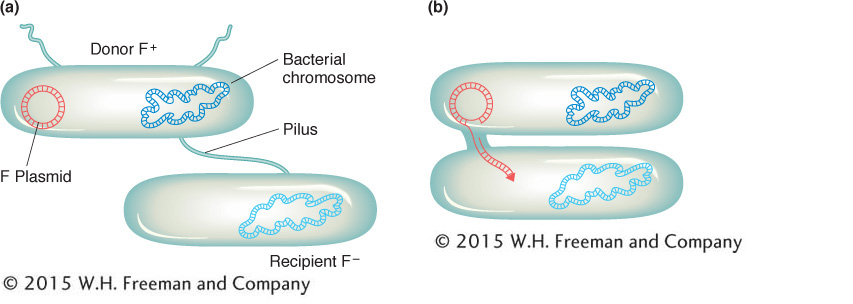
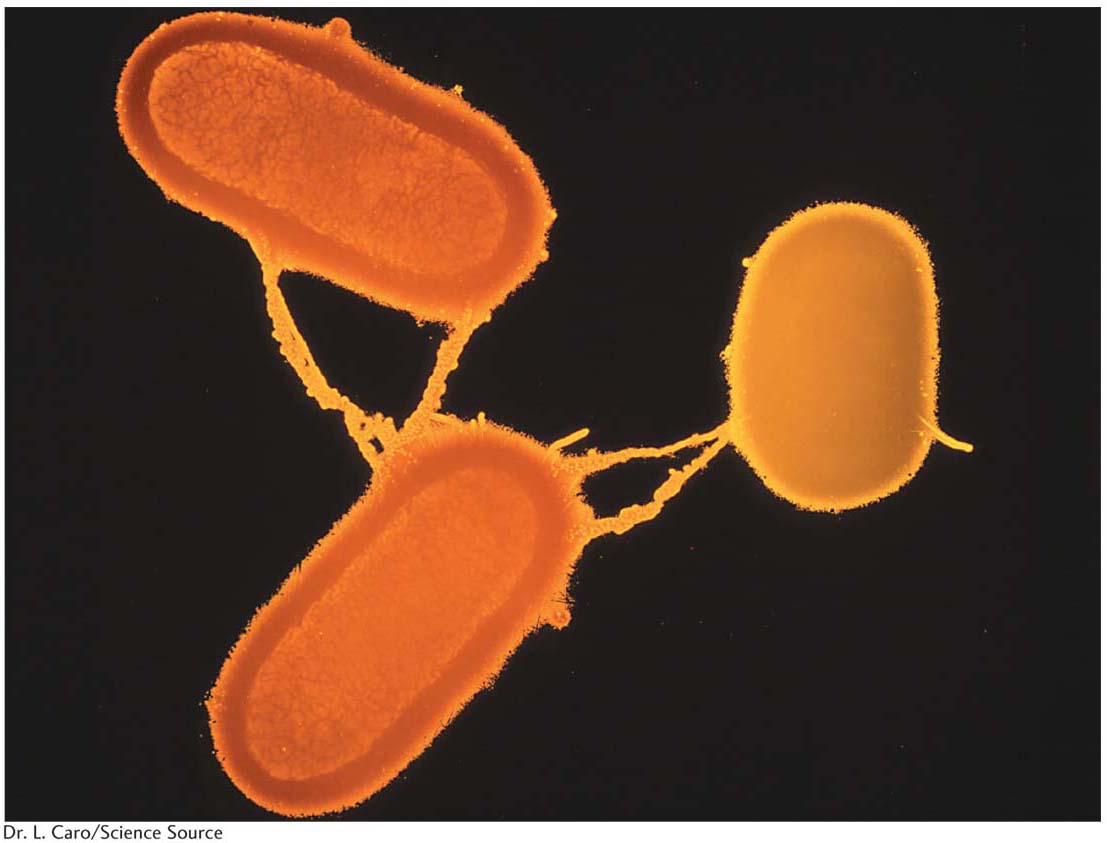
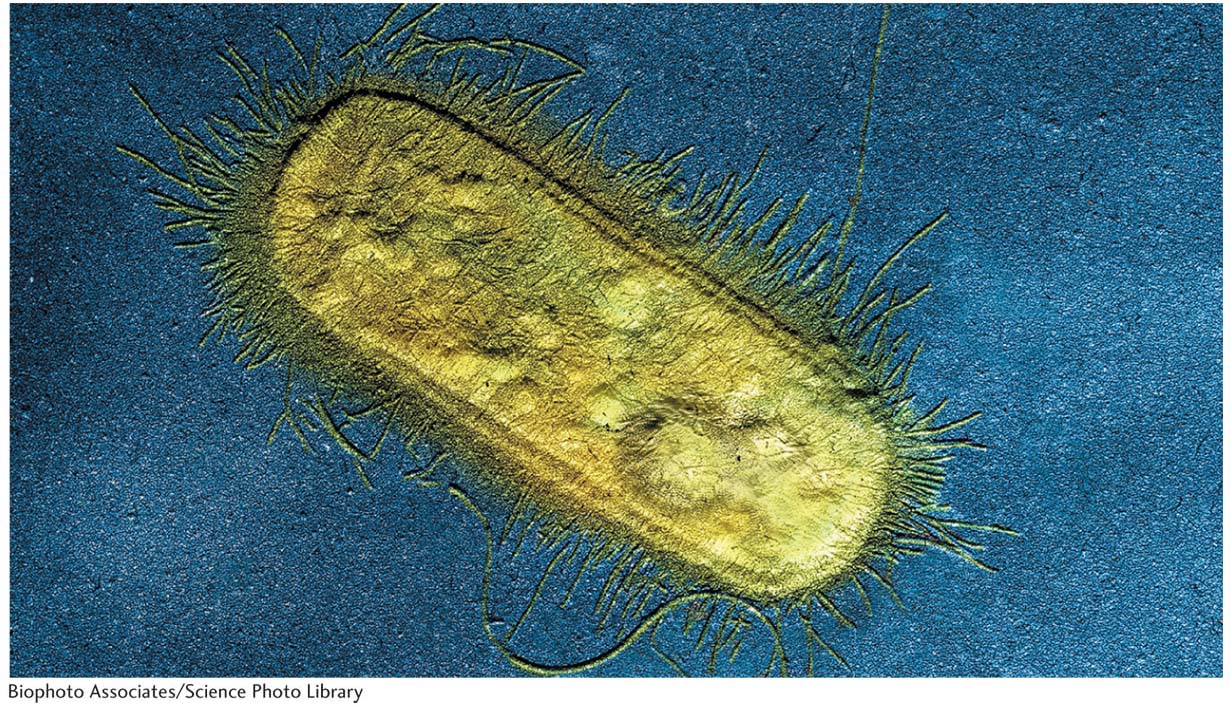
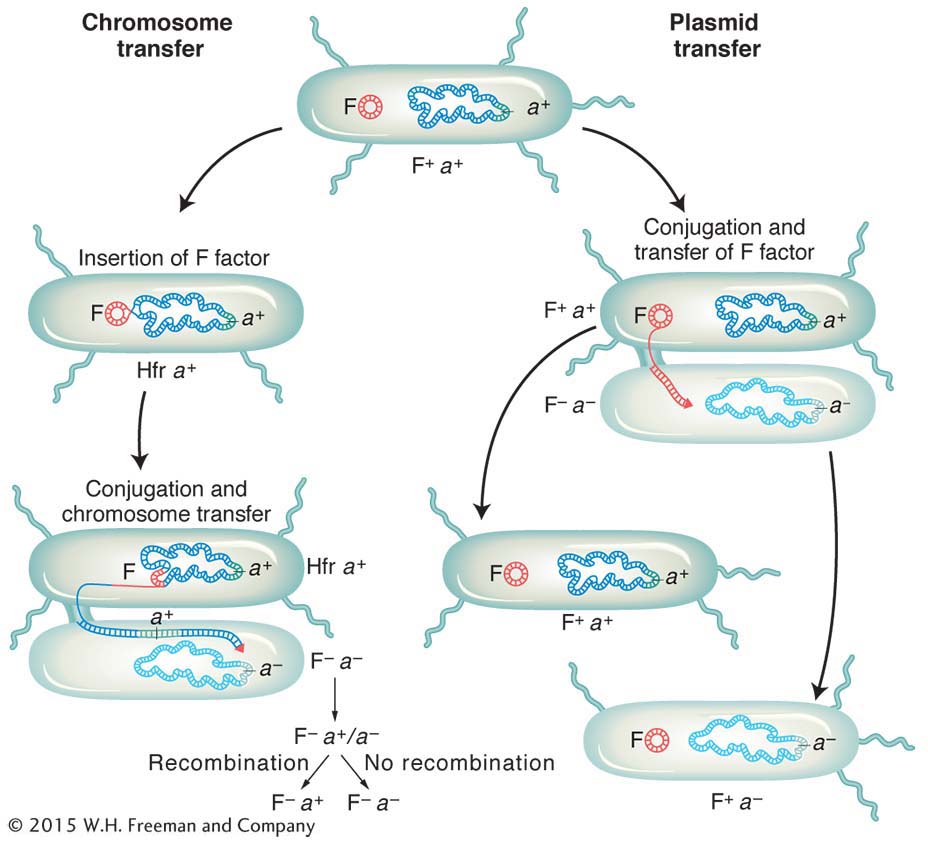
We now know much more about F. It is an example of a small, nonessential circular DNA molecule called a plasmid that can replicate in the cytoplasm independent of the host chromosome. Figure 5-8 shows how bacteria can transfer plasmids such as F. The F plasmid directs the synthesis of pili (sing., pilus), projections that initiate contact with a recipient (see Figures 5-7 and 5-8) and draw it closer. The F DNA in the donor cell makes a single-
Hfr strains
An important breakthrough came when Luca Cavalli-
On crossing with F− strains, this new strain produced 1000 times as many recombinants as a normal F+ strain. Cavalli-
Sforza designated this derivative an Hfr strain to symbolize its ability to promote a high frequency of recombination. In Hfr × F− crosses, virtually none of the F− parents were converted into F+ or into Hfr. This result is in contrast with F+ × F− crosses, in which, as we have seen, infectious transfer of F results in a large proportion of the F− parents being converted into F+.
Escherichia coli
The seventeenth-
The choice of E. coli was fortunate because it has proved to have many features suitable for genetic research, not the least of which is that it is easily obtained, given that it lives in the gut of humans and other animals. In the gut, it is a benign symbiont, but it occasionally causes urinary tract infections and diarrhea.
E. coli has a single circular chromosome 4.6 Mb in length. Of its 4000 intron-
E. coli is unicellular and grows by simple cell division. Because of its small size (~1 μm in length), E. coli can be grown in large numbers and subjected to intensive selection and screening for rare genetic events. E. coli research represents the beginning of “black box” reasoning in genetics: through the selection and analysis of mutants, the workings of the genetic machinery could be deduced even though it was too small to be seen. Phenotypes such as colony size, drug resistance, carbon-
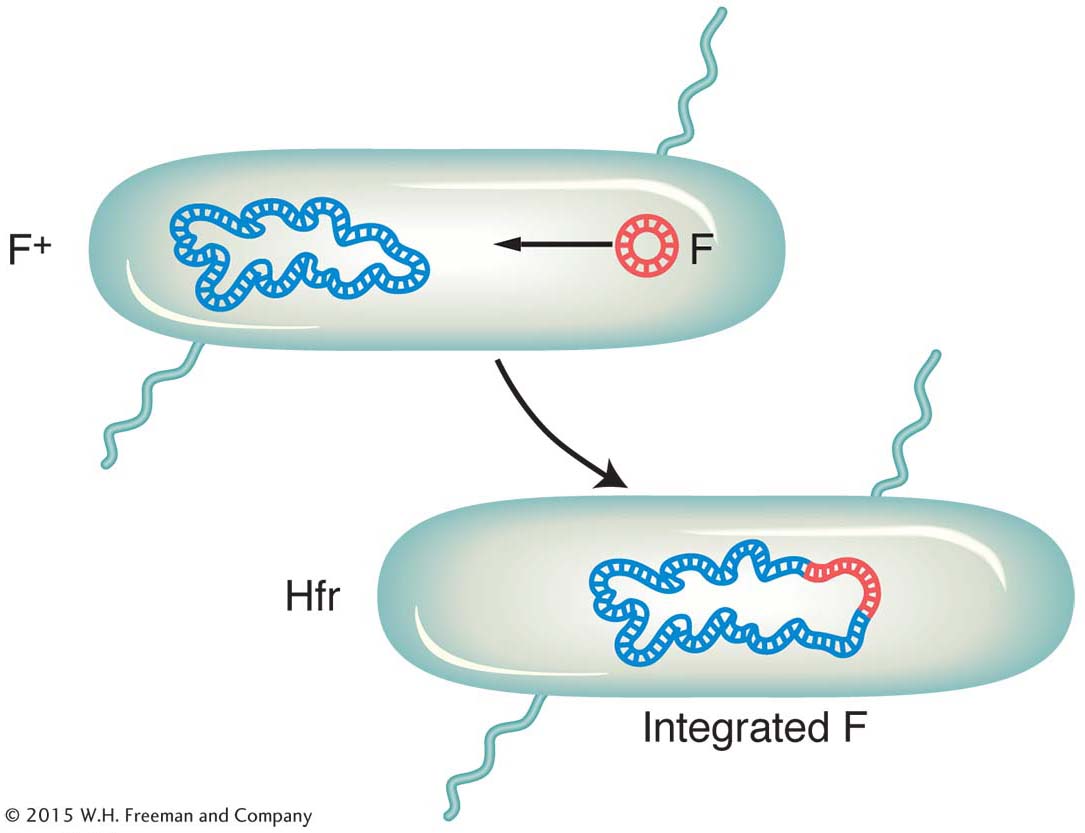
It became apparent that an Hfr strain results from the integration of the F factor into the chromosome, as pictured in Figure 5-9. We can now explain the first unusual property of Hfr strains. During conjugation, the F factor inserted in the chromosome efficiently drives part or all of that chromosome into the F− cell. The chromosomal fragment can then engage in recombination with the recipient chromosome. The rare recombinants observed by Lederberg and Tatum in F+ × F− crosses were due to the spontaneous, but rare, formation of Hfr cells in the F+ culture. Cavalli-
180
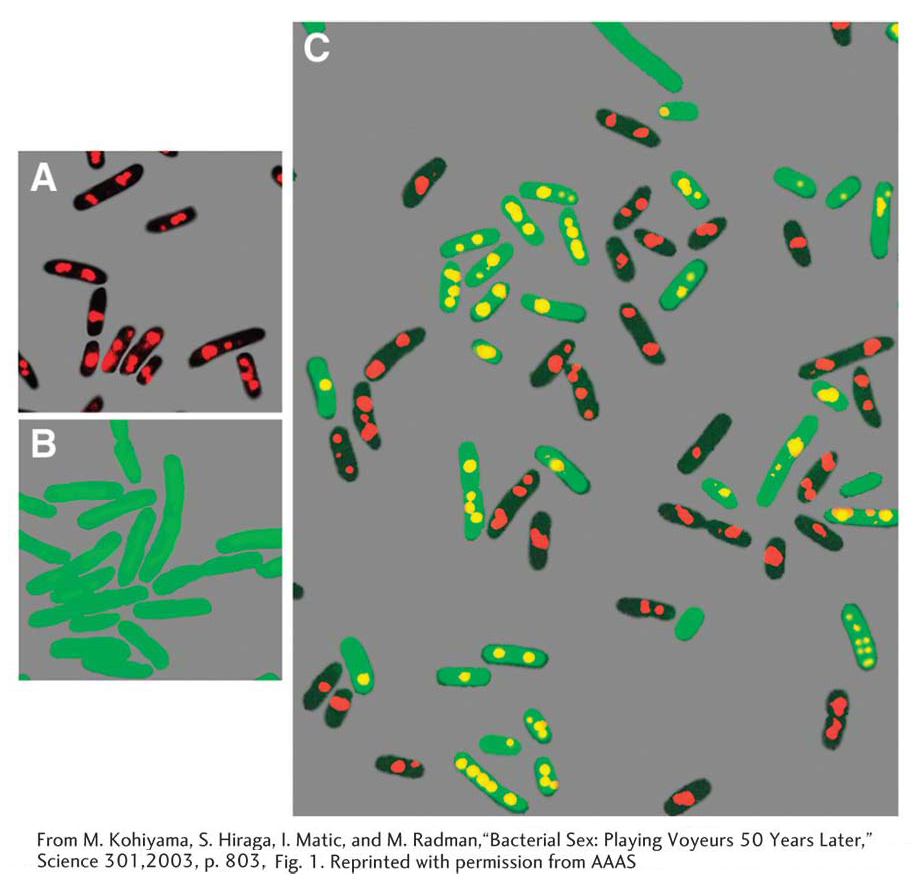
Does an Hfr cell die after donating its chromosomal material to an F− cell? The answer is no. Just like the F plasmid, the Hfr chromosome replicates and transfers a single strand to the F− cell during conjugation. That the transferred DNA is a single strand can be demonstrated visually with the use of special strains and antibodies, as shown in Figure 5-10. The replication of the chromosome ensures a complete chromosome for the donor cell after mating. The transferred strand is converted into a double helix in the recipient cell, and donor genes may become incorporated in the recipient’s chromosome through crossovers, creating a recombinant cell (Figure 5-11). If there is no recombination, the transferred fragments of DNA are simply lost in the course of cell division.
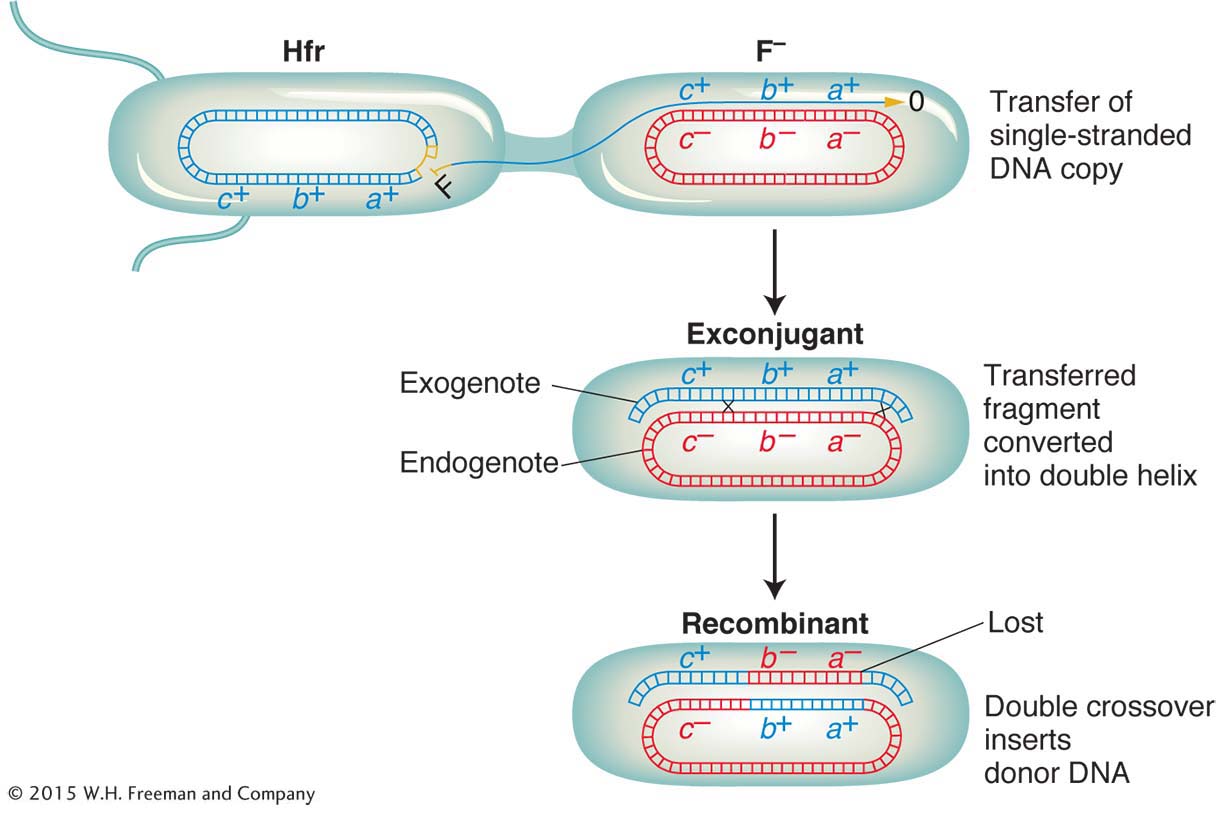
ANIMATED ART: Bacterial conjugation and recombination
181
Linear transmission of the Hfr genes from a fixed point A clearer view of the behavior of Hfr strains was obtained in 1957, when Elie Wollman and François Jacob investigated the pattern of transmission of Hfr genes to F− cells during a cross. They crossed
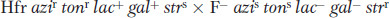
(Superscripts “r” and “s” stand for resistant and sensitive, respectively.) At specific times after mixing, they removed samples, which were each put in a kitchen blender for a few seconds to separate the mating cell pairs. This procedure is called interrupted mating. The sample was then plated onto a medium containing streptomycin to kill the Hfr donor cells, which bore the sensitivity allele strs. The surviving strr cells then were tested for the presence of alleles from the donor Hfr genome. Any strr cell bearing a donor allele must have taken part in conjugation; such cells are called exconjugants. The results are plotted in Figure 5-12a, showing a time course of entry of each donor allele azir, tonr, lac+, and gal+. Figure 5-12b portrays the transfer of Hfr alleles.
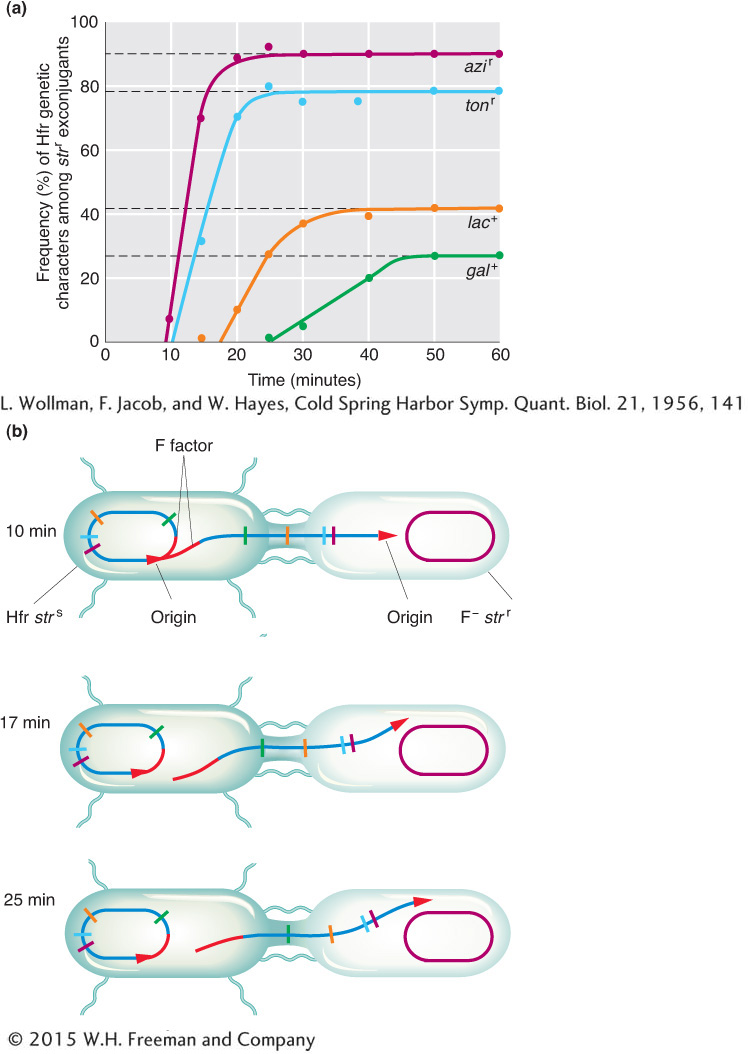
The key elements in these results are
Each donor allele first appears in the F− recipients at a specific time after mating began.
The donor alleles appear in a specific sequence.
Later donor alleles are present in fewer recipient cells.
182
Putting all these observations together, Wollman and Jacob deduced that, in the conjugating Hfr, single-
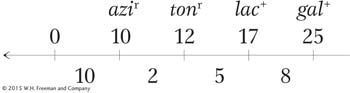
How can we explain the second unusual property of Hfr crosses, that F− exconjugants are rarely converted into Hfr or F+? When Wollman and Jacob allowed Hfr × F− crosses to continue for as long as 2 hours before disruption, they found that in fact a few of the exconjugants were converted into Hfr. In other words, the part of F that confers donor ability was eventually transmitted but at a very low frequency. The rareness of Hfr exconjugants suggested that the inserted F was transmitted as the last element of the linear chromosome. We can summarize the order of transmission with the following general type of map, in which the arrow indicates the direction of transfer, beginning with O:
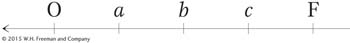
Thus, almost none of the F− recipients are converted, because the fertility factor is the last element transmitted and usually the transmission process will have stopped before getting that far.
KEY CONCEPT
The Hfr chromosome, originally circular, unwinds a copy of itself that is transferred to the F− cell in a linear fashion, with the F factor entering last.183
Inferring integration sites of F and chromosome circularity Wollman and Jacob went on to shed more light on how and where the F plasmid integrates to form an Hfr cell and, in doing so, deduced that the chromosome is circular. They performed interrupted-
|
Hfr strain |
|
|
H |
O thr pro lac pur gal his gly thi F |
|
1 |
O thr thi gly his gal pur lac pro F |
|
2 |
O pro thr thi gly his gal pur lac F |
|
3 |
O pur lac pro thr thi gly his gal F |
|
AB 312 |
O thi thr pro lac pur gal his gly F |
Each line can be considered a map showing the order of alleles on the chromosome. At first glance, there seems to be a random shuffling of genes. However, when some of the Hfr maps are inverted, the relation of the sequences becomes clear.
|
H |
F thi gly his gal pur lac pro thr O |
|
(written backward) |
|
|
1 |
O thr thi gly his gal pur lac pro F |
|
2 |
O pro thr thi gly his gal pur lac F |
|
3 |
O pur lac pro thr thi gly his gal F |
|
AB 312 |
F gly his gal pur lac pro thr thi O |
|
(written backward) |
|
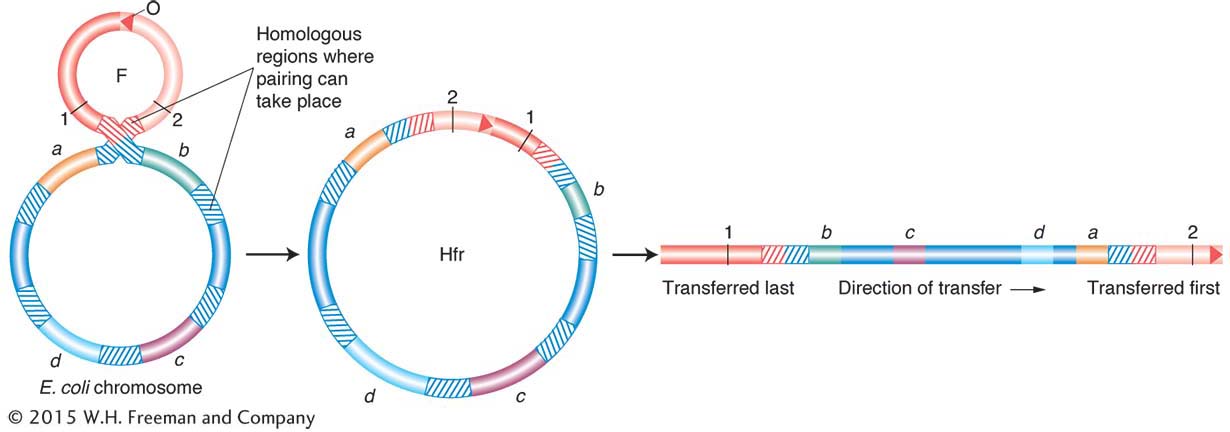
The relation of the sequences to one another is explained if each map is the segment of a circle. It was the first indication that bacterial chromosomes are circular. Furthermore, Allan Campbell proposed a startling hypothesis that accounted for the different Hfr maps. He proposed that, if F is a ring, then insertion might be by a simple crossover between F and the bacterial chromosome (Figure 5-13). That being the case, any of the linear Hfr chromosomes could be generated simply by the insertion of F into the ring in the appropriate place and orientation (Figure 5-14).
Several hypotheses—
One end of the integrated F factor would be the origin, where transfer of the Hfr chromosome begins. The terminus would be at the other end of F.
The orientation in which F is inserted would determine the order of entry of donor alleles. If the circle contains genes A, B, C, and D, then insertion between A and D would give the order ABCD or DCBA, depending on orientation. Check the different orientations of the insertions in Figure 5-14.
184
How is it possible for F to integrate at different sites? If F DNA had a region homologous to any of several regions on the bacterial chromosome, any one of them could act as a pairing region at which pairing could be followed by a crossover. These regions of homology are now known to be mainly segments of trans-
The fertility factor thus exists in two states:
The plasmid state: As a free cytoplasmic element, F is easily transferred to F− recipients.
The integrated state: As a contiguous part of a circular chromosome, F is transmitted only very late in conjugation.
The E. coli conjugation cycle is summarized in Figure 5-15.
Mapping of bacterial chromosomes
Broad-
Fine-
185
186
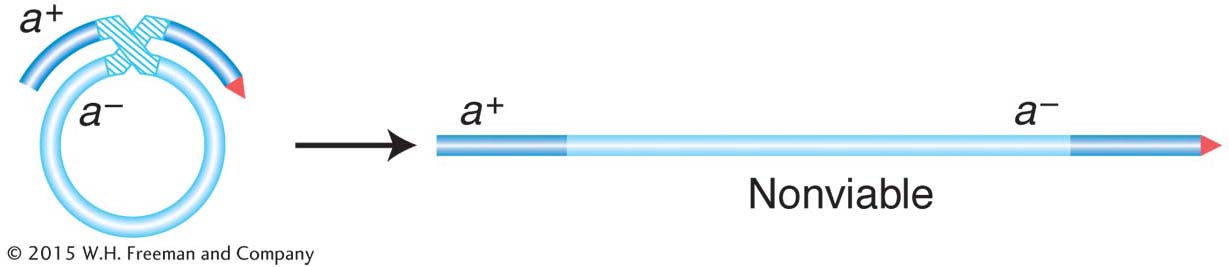
First, we need to understand some special features of the recombination event in bacteria. Recall that recombination does not take place between two whole genomes, as it does in eukaryotes. In contrast, it takes place between one complete genome, from the F− recipient cell, called the endogenote, and an incomplete one, derived from the Hfr donor cell and called the exogenote. The cell at this stage has two copies of one segment of DNA: one copy is part of the endogenote and the other copy is part of the exogenote. Thus, at this stage, the cell is a partial diploid, called a merozygote. Bacterial genetics is merozygote genetics. A single crossover in a merozygote would break the ring and thus not produce viable recombinants, as shown in Figure 5-16. To keep the circle intact, there must be an even number of crossovers. An even number of crossovers produces a circular, intact chromosome and a fragment. Although such recombination events are represented in a shorthand way as double crossovers, the actual molecular mechanism is somewhat different, more like an invasion of the endogenote by an internal section of the exogenote. The other product of the “double crossover,” the fragment, is generally lost in subsequent cell growth. Hence, only one of the reciprocal products of recombination survives. Therefore, another unique feature of bacterial recombination is that we must forget about reciprocal exchange products in most cases.
KEY CONCEPT
Recombination during conjugation results from a double-With this understanding, we can examine recombination mapping. Suppose that we want to calculate map distances separating three close loci: met, arg, and leu. To examine the recombination of these genes, we need “trihybrids,” exconjugants that have received all three donor markers. Assume that an interrupted-
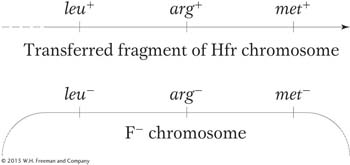
To obtain this merozygote, we must first select stable exconjugants bearing the last donor allele, which, in this case, is leu+. Why? In leu+ exconjugants, we know all three markers were transferred into the recipient because leu is the last donor allele. We also know that at least the leu+ marker was integrated into the endogenote. We want to know how often the other two markers were also integrated so that we can determine the number of recombination events in which arg+ or met+ was omitted due to double crossover.
The goal now is to count the frequencies of crossovers at different locations. Note that we now have a different situation from the analysis of interrupted conjugation. In mapping by interrupted conjugation, we measure the time of entry of individual loci; to be stably inherited, each marker has to recombine into the recipient chromosome by a double crossover spanning it. However, in the recombinant frequency analysis, we have specifically selected trihybrids as a starting point, and now we have to consider the various possible combinations of the three donor alleles that can be inserted by double crossing over in the various intervals. We know that leu+ must have entered and inserted because we selected it, but the leu+ recombinants that we select may or may not have incorporated the other donor markers, depending on where the double crossover took place. Hence, the procedure is to first select leu+ exconjugants and then isolate and test a large sample of them to see which of the other markers were integrated.
187
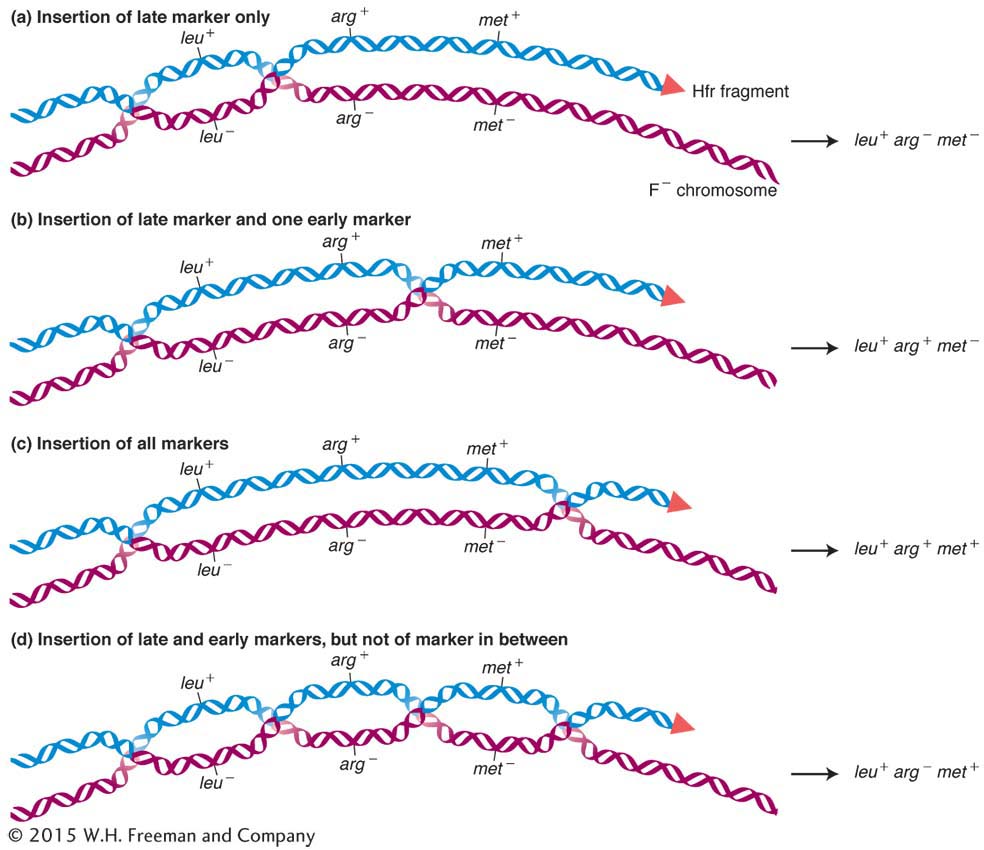
Let’s look at an example. In the cross Hfr met+ arg+ leu+ strs × F− met− arg− leu− strr, we would select leu+ recombinants and then examine them for the arg+ and met+ alleles, called the unselected markers. Figure 5-17 depicts the types of double-
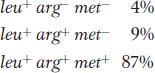
ANIMATED ART: Bacterial conjugation and mapping by recombination
188
The double crossovers needed to produce these genotypes are shown in Figure 5-17. The first two types are the key because they require a crossover between leu and arg in the first case and between arg and met in the second. Hence, the relative frequencies of these types correspond to the sizes of these two regions between the genes. We would conclude that the leu-
In a cross such as the one just described, one type of potential recombinants of genotype leu+ arg− met+ requires four crossovers instead of two (see the bottom of Figure 5-17). These recombinants are rarely recovered because their frequency is very low compared with that of the other types of recombinants.
F plasmids that carry genomic fragments
The F factor in Hfr strains is generally quite stable in its inserted position. However, occasionally an F factor cleanly exits from the chromosome by a reversal of the recombination process that inserted it in the first place. The two homologous pairing regions on either side repair, and a crossover takes place to liberate the F plasmid. However, sometimes the exit is not clean, and the plasmid carries with it a part of the bacterial chromosome. An F plasmid carrying bacterial genomic DNA is called an F′ (F prime) plasmid.
The first evidence of this process came from experiments in 1959 by Edward Adelberg and François Jacob. One of their key observations was of an Hfr in which the F factor was integrated near the lac+ locus. Starting with this Hfr lac+ strain, Jacob and Adelberg found an F+ derivative that, in crosses, transferred lac+ to F− lac− recipients at a very high frequency. (These transferrants could be detected by plating on medium containing lactose, on which only lac+ can grow.) The transferred lac+ is not incorporated into the recipient’s main chromosome, which we know retains the allele lac− because these F+ lac+ exconjugants occasionally gave rise to F− lac− daughter cells, at a frequency of 1 × 10–3. Thus, the genotype of these recipients appeared to be F′ lac+/F− lac−. In other words, the lac+ exconjugants seemed to carry an F′ plasmid with a piece of the donor chromosome incorporated. The origin of this F′ plasmid is shown in Figure 5-18. Note that the faulty excision occurs because there is another homologous region nearby that pairs with the original. The F′ in our example is called F′ lac because the piece of host chromosome that it picked up has the lac gene on it. F′ factors have been found carrying many different chromosomal genes and have been named accordingly. For example, F′ factors carrying gal or trp are called F′ gal and F′ trp, respectively. Because F lac+/F− lac− cells are lac+ in phenotype, we know that lac+ is dominant over lac−.
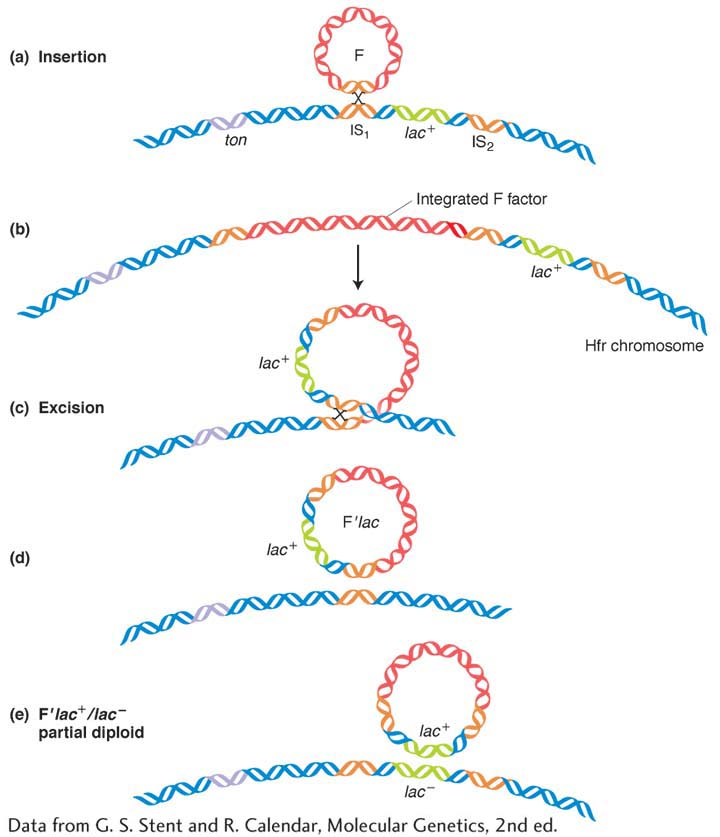
Partial diploids made with the use of F′ strains are useful for some aspects of routine bacterial genetics, such as the study of dominance or of allele interaction. Some F′ strains can carry very large parts (as much as one-
KEY CONCEPT
The DNA of an F′ plasmid is part F factor and part bacterial genome. Like F plasmids, F′ plasmids transfer rapidly. They can be used to establish partial diploids for studies of bacterial dominance and allele interaction.R plasmids
An alarming property of pathogenic bacteria first came to light through studies in Japanese hospitals in the 1950s. Bacterial dysentery is caused by bacteria of the genus Shigella. This bacterium was initially sensitive to a wide array of antibiotics that were used to control the disease. In the Japanese hospitals, however, Shigella isolated from patients with dysentery proved to be simultaneously resistant to many of these drugs, including penicillin, tetracycline, sulfanilamide, streptomycin, and chloramphenicol. This resistance to multiple drugs was inherited as a single genetic package, and it could be transmitted in an infectious manner—
189
From the point of view of the geneticist, however, the mechanism has proved interesting and is useful in genetic engineering. The vectors carrying these multiple resistances proved to be another group of plasmids called R plasmids. They are transferred rapidly on cell conjugation, much like the F plasmid in E. coli.
In fact, the R plasmids in Shigella proved to be just the first of many similar genetic elements to be discovered. All exist in the plasmid state in the cytoplasm.
190
|
Characteristic |
Plasmid examples |
|---|---|
|
Fertility |
F, R1, Col |
|
Bacteriocin production |
Col E1 |
|
Heavy- |
R6 |
|
Enterotoxin production |
Ent |
|
Metabolism of camphor |
Cam |
|
Tumorigenicity in plants |
T1 (in Agrobacterium tumefaciens) |
These elements have been found to carry many different kinds of genes in bacteria. Table 5-2 shows some of the characteristics that can be borne by plasmids. Figure 5-19 shows an example of a well-
Engineered derivatives of R plasmids, such as pBR 322 and pUC (see Chapter 10), have become the preferred vectors for the molecular cloning of the DNA of all organisms. The genes on an R plasmid that confer resistance can be used as markers to keep track of the movement of the vectors between cells.
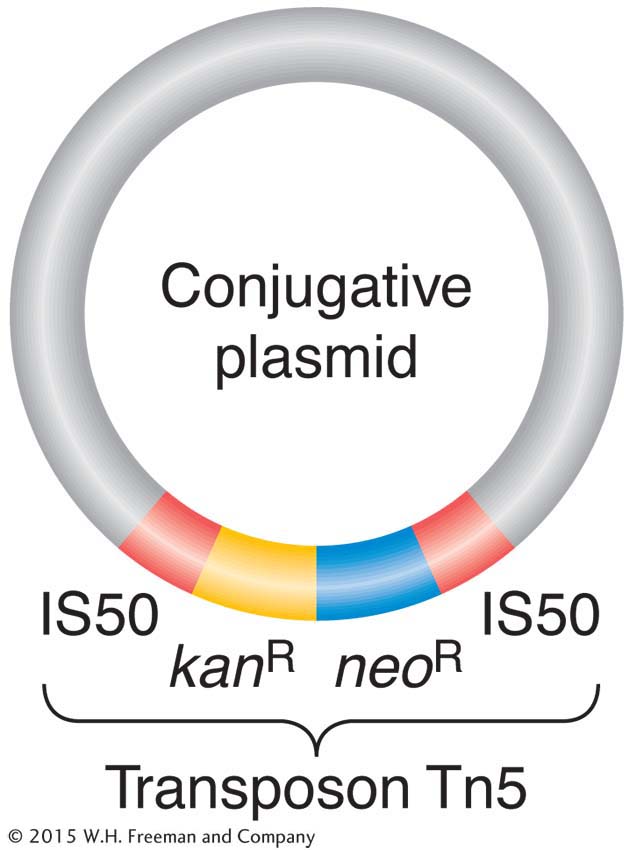
On R plasmids, the alleles for antibiotic resistance are often contained within a unit called a transposon (Figure 5-20). Transposons are unique segments of DNA that can move around to different sites in the genome, a process called transposition. (The mechanisms for transposition, which occurs in most species studied, will be detailed in Chapter 15.) When a transposon in the genome moves to a new location, it can occasionally embrace between its ends various types of genes, including alleles for drug resistance, and carry them along to their new locations as passengers. Sometimes, a transposon carries a drug-
191