5.5 Transduction
Some phages are able to pick up bacterial genes and carry them from one bacterial cell to another, a process known as transduction. Thus, transduction joins the battery of modes of transfer of genomic material between bacteria—
Discovery of transduction
In 1951, Joshua Lederberg and Norton Zinder were testing for recombination in the bacterium Salmonella typhimurium by using the techniques that had been successful with E. coli. The researchers used two different strains: one was phe− trp−tyr−, and the other was met− his−. We won’t worry about the nature of these alleles except to note that all are auxotrophic. When either strain was plated on a minimal medium, no wild-
However, in this case, the researchers also recovered recombinants from a U-
To understand the process of transduction, we need to distinguish two types of phage cycle. Virulent phages are those that immediately lyse and kill the host. Temperate phages can remain within the host cell for a period without killing it. Their DNA either integrates into the host chromosome to replicate with it or replicates separately in the cytoplasm, as does a plasmid. A phage integrated into the bacterial genome is called a prophage. A bacterium harboring a quiescent phage is described as lysogenic and is itself called a lysogen. Occasionally, the quiescent phage in a lysogenic bacterium becomes active, replicates itself, and causes the spontaneous lysis of its host cell. A resident temperate phage confers resistance to infection by other phages of that type.
There are two kinds of transduction: generalized and specialized. Generalized transducing phages can carry any part of the bacterial chromosome, whereas specialized transducing phages carry only certain specific parts.
KEY CONCEPT
Virulent phages cannot become prophages; they replicate and lyse a cell immediately. Temperate phages can exist within the bacterial cell as prophages, allowing their hosts to survive as lysogenic bacteria; they are also capable of occasional bacterial lysis.197
Generalized transduction
By what mechanisms can a phage carry out generalized transduction? In 1965, H. Ikeda and J. Tomizawa threw light on this question in some experiments on the E. coli phage P1. They found that, when a donor cell is lysed by P1, the bacterial chromosome is broken up into small pieces. Occasionally, the newly forming phage particles mistakenly incorporate a piece of the bacterial DNA into a phage head in place of phage DNA. This event is the origin of the transducing phage.
A phage carrying bacterial DNA can infect another cell. That bacterial DNA can then be incorporated into the recipient cell’s chromosome by recombination (Figure 5-29). Because genes on any of the cut-

Phages P1 and P22 both belong to a phage group that shows generalized transduction. P22 DNA inserts into the host chromosome, whereas P1 DNA remains free, like a large plasmid. However, both transduce by faulty head stuffing.
Generalized transduction can be used to obtain bacterial linkage information when genes are close enough that the phage can pick them up and transduce them in a single piece of DNA. For example, suppose that we wanted to find the linkage distance between met and arg in E. coli. We could grow phage P1 on a donor met+ arg+ strain and then allow P1 phages from lysis of this strain to infect a met− arg− strain. First, one donor allele is selected, say, met+. Then the percentage of met+ colonies that are also arg+ is measured. Strains transduced to both met+ and arg+ are called cotransductants. The greater the cotransduction frequency, the closer two genetic markers must be (the opposite of most mapping measurements). Linkage values are usually expressed as cotransduction frequencies (Figure 5-30).
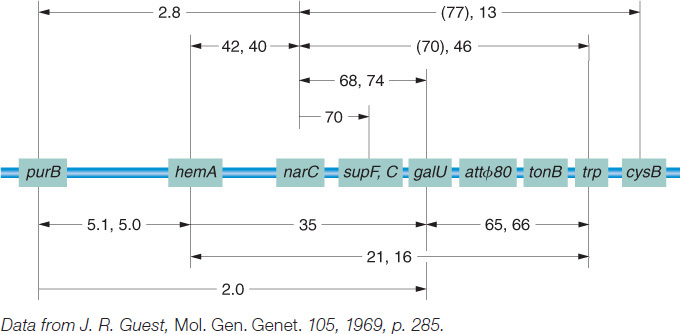
198
By using an extension of this approach, we can estimate the size of the piece of host chromosome that a phage can pick up, as in the following type of experiment, which uses P1 phage:
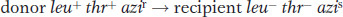
In this experiment, P1 phage grown on the leu+ thr+ azir donor strain infect the leu− thr− azis recipient strain. The strategy is to select one or more donor alleles in the recipient and then test these transductants for the presence of the unselected alleles. Results are outlined in Table 5-3. Experiment 1 in Table 5-3 tells us that leu is relatively close to azi and distant from thr, leaving us with two possibilities:

|
Experiment |
Selected marker |
Unselected markers |
|---|---|---|
|
1 |
leu+ |
50% are azir; 2% are thr+ |
|
2 |
thr+ |
3% are leu+; 0% are arir |
|
3 |
leu+ and thr+ |
0% are azir |
Experiment 2 tells us that leu is closer to thr than azi is, and so the map must be

By selecting for thr+ and leu+ together in the transducing phages in experiment 3, we see that the transduced piece of genetic material never includes the azi locus because the phage head cannot carry a fragment of DNA that big. P1 can only cotransduce genes less than approximately 1.5 minutes apart on the E. coli chromosome map.
Specialized transduction
A generalized transducer, such as phage P22, picks up fragments of broken host DNA at random. How are other phages, which act as specialized transducers, able to carry only certain host genes to recipient cells? The short answer is that a specialized transducer inserts into the bacterial chromosome at one position only. When it exits, a faulty outlooping occurs (similar to the type that produces F′ plasmids). Hence, it can pick up and transduce only genes that are close by.
199
The prototype of specialized transduction was provided by studies undertaken by Joshua and Esther Lederberg on a temperate E. coli phage called lambda (λ). Phage λ has become the most intensively studied and best-
Behavior of the prophage Phage λ has unusual effects when cells lysogenic for it are used in crosses. In the cross of an uninfected Hfr with a lysogenic F− recipient [Hfr × F−(λ)], lysogenic F− exconjugants with Hfr genes are readily recovered. However, in the reciprocal cross Hfr(λ) × F−, the early genes from the Hfr chromosome are recovered among the exconjugants, but recombinants for late genes are not recovered. Furthermore, lysogenic exconjugants are almost never recovered from this reciprocal cross. What is the explanation? The observations make sense if the λ prophage is behaving as a bacterial gene locus behaves (that is, as part of the bacterial chromosome). Thus, in the Hfr(λ) × F− cross, the prophage would enter the F− cell at a specific time corresponding to its position in the chromosome. Earlier genes are recovered because they enter before the prophage. Later genes are not recovered because lysis destroys the recipient cell. In interrupted-
In an Hfr(λ) × F− cross, the entry of the λ prophage into the cell immediately triggers the prophage into a lytic cycle; this process is called zygotic induction (Figure 5-31). However, in the cross of two lysogenic cells Hfr(λ) × F−(λ), there is no zygotic induction. The presence of any prophage prevents another infecting virus from causing lysis. This is because the prophage produces a cytoplasmic factor that represses the multiplication of the virus. (The phage-
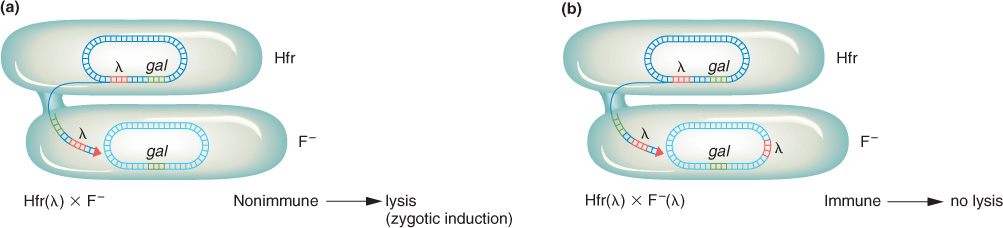
λ insertion The interrupted-
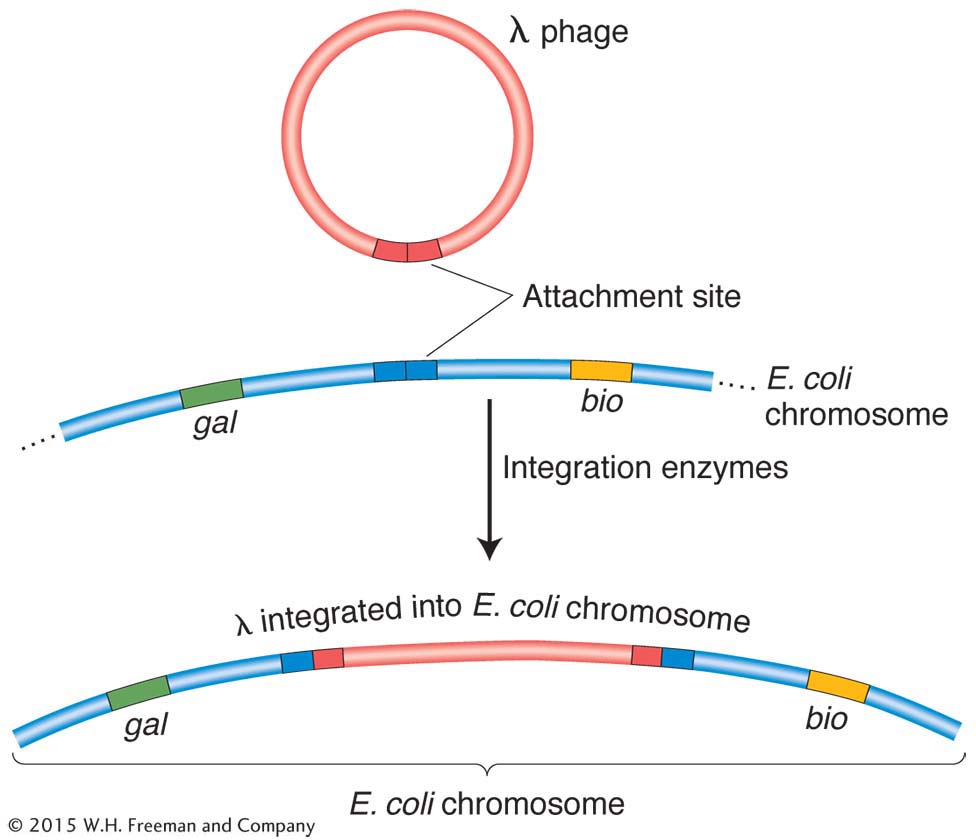
An attraction of Campbell’s proposal is that from it follow predictions that geneticists can test. For example, integration of the prophage into the E. coli chromosome should increase the genetic distance between flanking bacterial genes, as can be seen in Figure 5-32 for gal and bio. In fact, studies show that lysogeny does increase time-
200
Mechanism of specialized transduction
As a prophage, λ always inserts between the gal region and the bio region of the host chromosome (Figure 5-33), and, in transduction experiments, as expected, λ can transduce only the gal and bio genes.
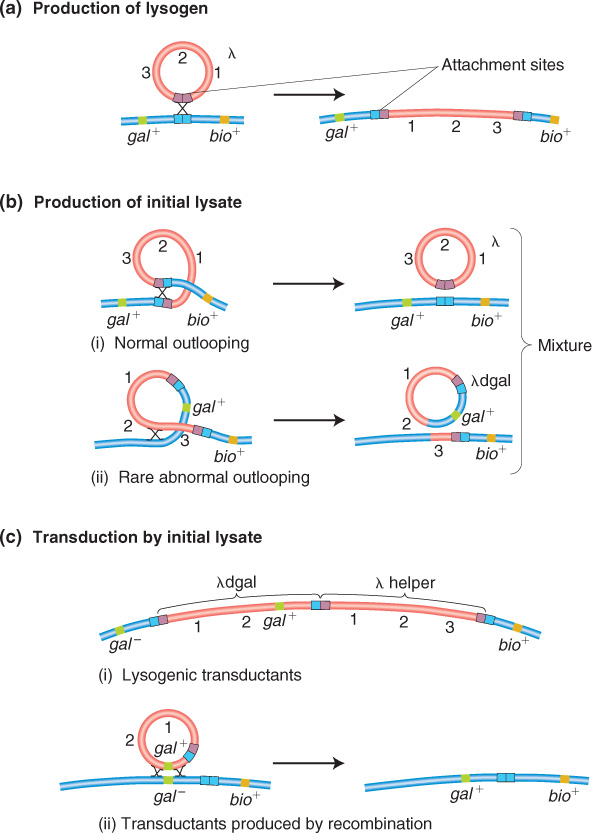
How does λ carry away neighboring genes? The explanation lies, again, in an imperfect reversal of the Campbell insertion mechanism, like that for F′ formation. The recombination event between specific regions of λ and the bacterial chromosome is catalyzed by a specialized phage-
KEY CONCEPT
Transduction occurs when newly forming phages acquire host genes and transfer them to other bacterial cells. Generalized transduction can transfer any host gene. It occurs when phage packaging accidentally incorporates bacterial DNA instead of phage DNA. Specialized transduction is due to faulty outlooping of the prophage from the bacterial chromosome, and so the new phage includes both phage and bacterial genes. The transducing phage can transfer only specific host genes.201