7.3 Semiconservative Replication
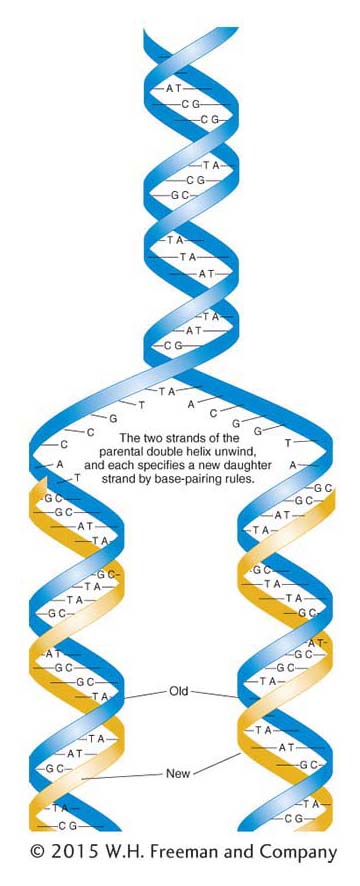
Figure 7-11: Semiconservative DNA replication
Figure 7-11: The semiconservative model of DNA replication proposed by Watson and Crick is based on the hydrogen-bonded specificity of the base pairs. Parental strands, shown in blue, serve as templates for polymerization. The newly polymerized strands, shown in gold, have base sequences that are complementary to their respective templates.
The copying mechanism to which Watson and Crick referred is called semiconservative replication and is diagrammed in Figure 7-11. The sugar–phosphate backbones are represented by thick ribbons, and the sequence of base pairs is random. Let’s imagine that the double helix is analogous to a zipper that unzips, starting at one end. We can see that, if this zipper analogy is valid, the unwinding of the two strands will expose single bases on each strand. Each exposed base has the potential to pair with free nucleotides in solution. Because the DNA structure imposes strict pairing requirements, each exposed base will pair only with its complementary base, A with T and G with C. Thus, each of the two single strands will act as a template, or mold, to direct the assembly of complementary bases to re-form a double helix identical with the original. The newly added nucleotides are assumed to come from a pool of free nucleotides that must be present in the cell.
If this model is correct, then each daughter molecule should contain one parental nucleotide chain and one newly synthesized nucleotide chain. However, a little thought shows that there are at least three different ways in which a parental DNA molecule might be related to the daughter molecules. These hypothetical modes of replication are called semiconservative (the Watson–Crick model), conservative, and dispersive (Figure 7-12). In semiconservative replication, the double helix of each daughter DNA molecule contains one strand from the original DNA molecule and one newly synthesized strand. In conservative replication, the parent DNA molecule is conserved, and a single daughter double helix is produced consisting of two newly synthesized strands. In dispersive replication, daughter molecules consist of strands each containing segments of both parental DNA and newly synthesized DNA.
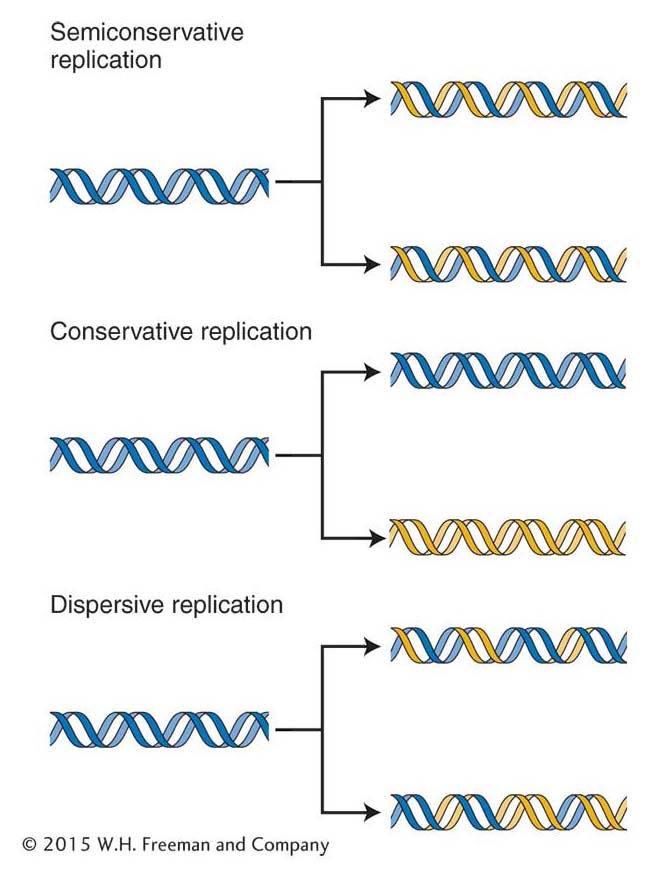
Figure 7-12: Three alternative models for DNA replication
Figure 7-12: Of three alternative models for DNA replication, the Watson-Crick model of DNA structure would produce the first (semiconservative) model. Gold lines represent the newly synthesized strands.
Meselson–Stahl experiment
The first problem in understanding DNA replication was to figure out whether the mechanism of replication was semiconservative, conservative, or dispersive. In 1958, two young scientists, Matthew Meselson and Franklin Stahl, set out to discover which of these possibilities correctly described DNA replication. Their idea was to allow parental DNA molecules containing nucleotides of one density to replicate in medium containing nucleotides of different density. If DNA replicated semiconservatively, the daughter molecules should be half old and half new and therefore of intermediate density.
To carry out their experiment, Meselson and Stahl grew E. coli cells in a medium containing the heavy isotope of nitrogen (15N) rather than the normal light (14N) form. This isotope was inserted into the nitrogen bases, which then were incorporated into newly synthesized DNA strands. After many cell divisions in 15N, the DNA of the cells were well labeled with the heavy isotope. The cells were then removed from the 15N medium and put into a 14N medium; after one and two cell divisions, samples were taken and the DNA was isolated from each sample.
Meselson and Stahl were able to distinguish DNA of different densities because the molecules can be separated from one another by a procedure called cesium chloride gradient centrifugation. If cesium chloride (CsCl) is spun in a centrifuge at tremendously high speeds (50,000 rpm) for many hours, the cesium and chloride ions tend to be pushed by centrifugal force toward the bottom of the tube. Ultimately, a gradient of ions is established in the tube, with the highest ion concentration, or density, at the bottom. DNA centrifuged with the cesium chloride forms a band at a position identical with its density in the gradient (Figure 7-13). DNA of different densities will form bands at different places. Cells initially grown in the heavy isotope 15N showed DNA of high density. This DNA is shown in blue at the left-hand side of Figure 7-13a. After growing these cells in the light isotope 14N for one generation, the researchers found that the DNA was of intermediate density, shown half blue (15N) and half gold (14N) in the middle of Figure 7-13a. Note that Meselson and Stahl continued the experiment through two E. coli generations so that they could distinguish semiconservative replication from dispersive. After two generations, both intermediate- and low-density DNA was observed (right-hand side of Figure 7-13a), precisely as predicted by Watson–Crick’s semiconservative replication model.
KEY CONCEPT
DNA is replicated by the unwinding of the two strands of the double helix and the building up of a new complementary strand on each of the separated strands of the original double helix.
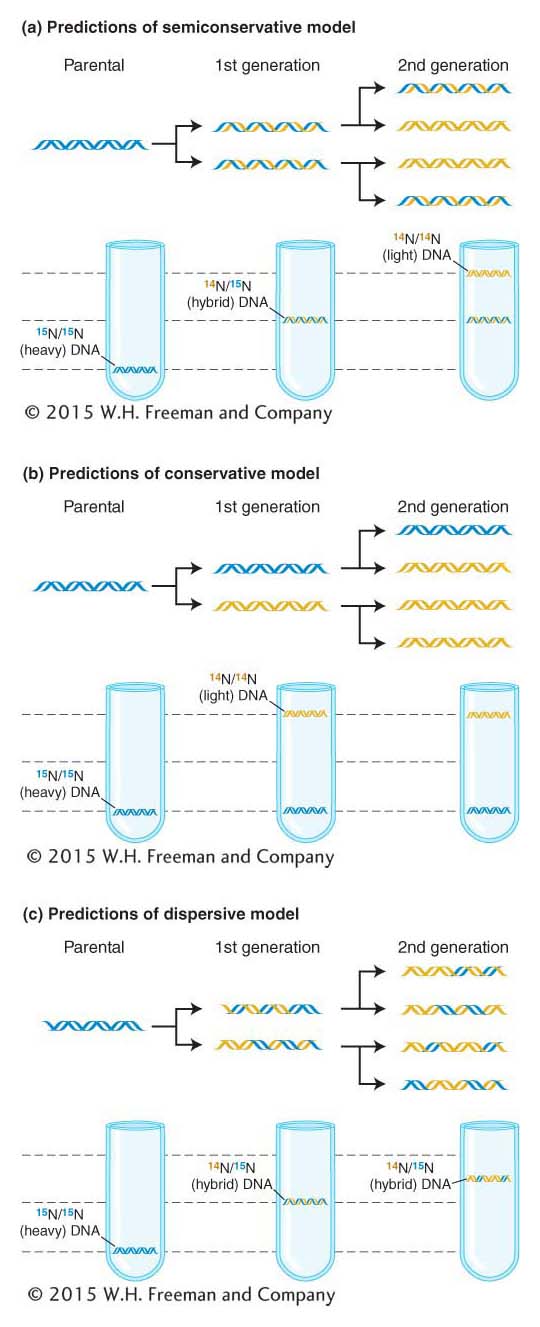
Figure 7-13: DNA is copied by semiconservative replication
Figure 7-13: The Meselson–Stahl experiment demonstrates that DNA is copied by semiconservative replication. DNA centrifuged in a cesium chloride (CsCl) gradient will form bands according to its density. (a) When the cells grown in 15N are transferred to a 14N medium, the first generation produces a single intermediate DNA band and the second generation produces two bands: one intermediate and one light. This result matches the predictions of the semiconservative model of DNA replication. (b and c) The results predicted for conservative and dispersive replication, shown here, were not found.
The replication fork
Another prediction of the Watson–Crick model of DNA replication is that a replication zipper, or fork, will be found in the DNA molecule during replication. This fork is the location at which the double helix is unwound to produce the two single strands that serve as templates for copying. In 1963, John Cairns tested this prediction by allowing replicating DNA in bacterial cells to incorporate tritiated thymidine ([3H]thymidine)—the thymine nucleotide labeled with a radioactive hydrogen isotope called tritium. Theoretically, each newly synthesized daughter molecule should then contain one radioactive (“hot”) strand (with 3H) and another nonradioactive (“cold”) strand. After varying intervals and varying numbers of replication cycles in a “hot” medium, Cairns carefully lysed the bacteria and allowed the cell contents to settle onto grids designed for electron microscopy. Finally, Cairns covered the grid with photographic emulsion and exposed it in the dark for 2 months. This procedure, called autoradiography, allowed Cairns to develop a picture of the location of 3H in the cell material. As 3H decays, it emits a beta particle (an energetic electron). The photographic emulsion detects a chemical reaction that takes place wherever a beta particle strikes the emulsion. The emulsion can then be developed like a photographic print so that the emission track of the beta particle appears as a black spot or grain.
After one replication cycle in [3H]thymidine, a ring of dots appeared in the autoradiograph. Cairns interpreted this ring as a newly formed radioactive strand in a circular daughter DNA molecule, as shown in Figure 7-14a. It is thus apparent that the bacterial chromosome is circular—a fact that also emerged from genetic analysis described earlier (see Chapter 5). In the second replication cycle, the forks predicted by the model were indeed seen. Furthermore, the density of grains in the three segments was such that the interpretation shown in Figure 7-14b could be made: the thick curve of dots cutting through the interior of the circle of DNA would be the newly synthesized daughter strand, this time consisting of two radioactive strands. Cairns saw all sizes of these moon-shaped, autoradiographic patterns, corresponding to the progressive movement of the replication forks, around the ring.
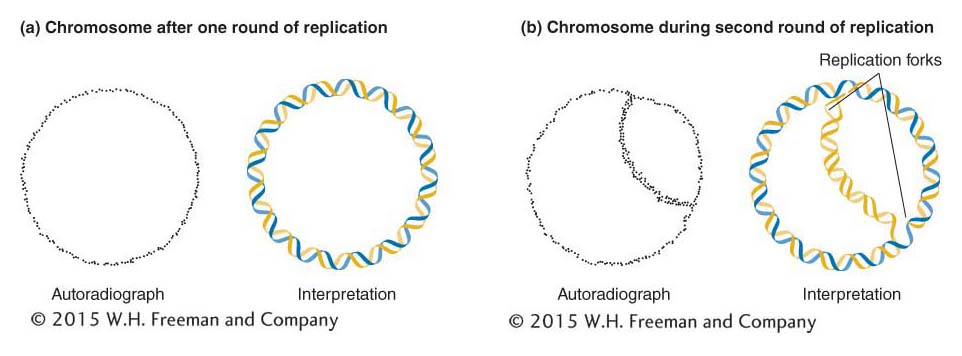
Figure 7-14: A replicating bacterial chromosome
Figure 7-14: A replicating bacterial chromosome has two replication forks. (a) Left: Autoradiograph of a bacterial chromosome after one replication in tritiated thymidine. According to the semiconservative model of replication, one of the two strands should be radioactive. Right: Interpretation of the autoradiograph. The gold helix represents the tritiated strand. (b) Left: Autoradiograph of a bacterial chromosome in the second round of replication in tritiated (3H) thymidine. In this molecule, the newly replicated double helix that crosses the circle could consist of two radioactive strands (if the parental strand were the radioactive one). Right: The double thickness of the radioactive tracing on the autoradiogram appears to confirm the interpretation shown here.
DNA polymerases
A problem confronted by scientists was to understand just how the bases are brought to the double-helix template. Although scientists suspected that enzymes played a role, that possibility was not proved until 1959, when Arthur Kornberg isolated DNA polymerase from E. coli and demonstrated its enzymatic activity in vitro. This enzyme adds deoxyribonucleotides to the 3′ end of a growing nucleotide chain, using for its template a single strand of DNA that has been exposed by localized unwinding of the double helix (Figure 7-15). The substrates for DNA polymerase are the triphosphate forms of the deoxyribonucleotides, dATP, dGTP, dCTP, and dTTP. The addition of each base to the growing polymer is accompanied by the removal of two of the three phosphates in the form of pyrophosphate (PPi). The energy produced by cleaving this high-energy bond and the subsequent hydrolysis of pyrophosphate to two inorganic phosphate molecules helps drive the endergonic process of building a DNA polymer.
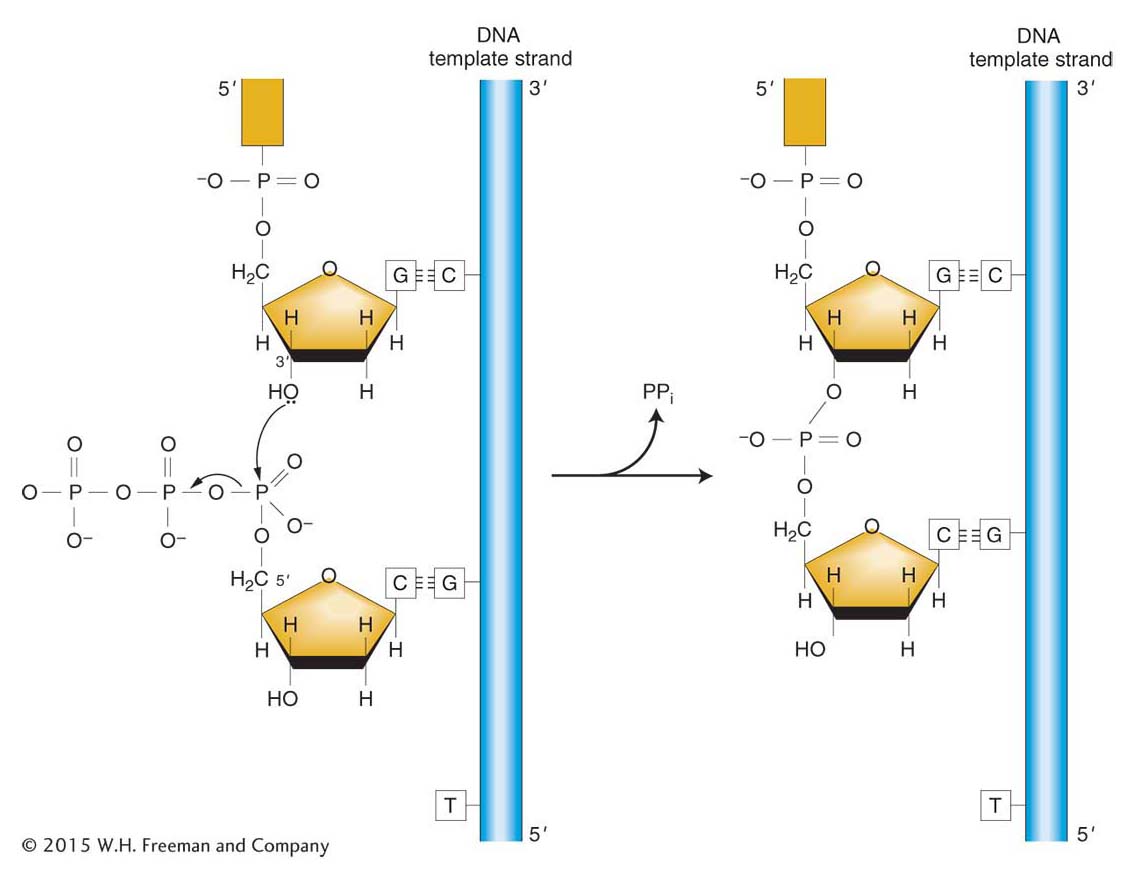
Figure 7-15: Reaction catalyzed by DNA polymerase
Figure 7-15: DNA polymerase catalyzes the chain-elongation reaction. Energy for the reaction comes from breaking the high-energy phosphate bond of the triphosphate substrate. ANIMATED ART: The nucleotide polymerization process
There are now known to be five DNA polymerases in E. coli. The first enzyme that Kornberg purified is now called DNA polymerase I, or pol I. This enzyme has three activities, which appear to be located in different parts of the molecule:
a polymerase activity, which catalyzes chain growth in the 5′-to-3′ direction;
a 3′-to-5′ exonuclease activity, which removes mismatched bases; and
a 5′-to-3′ exonuclease activity, which degrades single strands of DNA or RNA.
We will return to the significance of the two exonuclease activities later in this chapter.
Although pol I has a role in DNA replication (see next section), some scientists suspected that it was not responsible for the majority of DNA synthesis because it was too slow (~20 nucleotides/second) and too abundant (~400 molecules/cell) and because it dissociated from the DNA after incorporating from only 20 to 50 nucleotides. In 1969, John Cairns and Paula DeLucia settled this matter when they demonstrated that an E. coli strain harboring a mutation in the gene that encodes DNA pol I was still able to grow normally and replicate its DNA. They concluded that another DNA polymerase, now called pol III, catalyzes DNA synthesis at the replication fork.




