7.7 Telomeres and Telomerase: Replication Termination
Replication of the linear DNA molecule in a eukaryotic chromosome proceeds in both directions from numerous replication origins, as shown in Figure 7-23. This process replicates most of the chromosomal DNA, but there is an inherent problem in replicating the two ends of linear DNA molecules, the regions called telomeres. Continuous synthesis on the leading strand can proceed right up to the very tip of the template. However, lagging-strand synthesis requires primers ahead of the process; so, when the last primer is removed, sequences are missing at the end of that strand. As a consequence, a single-stranded tip remains in one of the daughter DNA molecules (Figure 7-26). If the daughter chromosome with this DNA molecule were replicated again, the strand missing sequences at the end would become a shortened double-stranded molecule after replication. At each subsequent replication cycle, the telomere would continue to shorten, until eventually essential coding information would be lost.
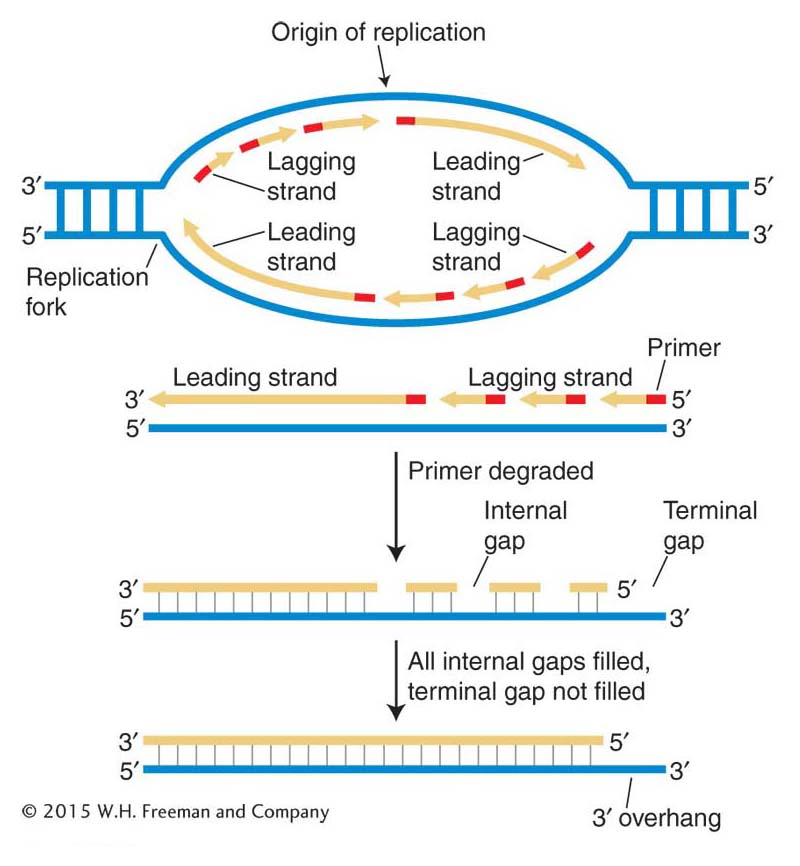
Figure 7-26: The replication problem at chromosome ends
Figure 7-26: Top: The replication of each Okazaki fragment on the lagging strand begins with the insertion of a primer. Bottom: The fate of the bottom strand in the transcription bubble. When the primer for the last Okazaki fragment of the lagging strand is removed, there is no way to fill the gap by conventional replication. A shortened chromosome would result when the chromosome containing the gap was replicated.
Cells have evolved a specialized system to prevent this loss. The solution involves the addition of multiple copies of a simple noncoding sequence to the DNA at the chromosome tips. Thus, every time a chromosome is duplicated, it is shortened and only these repeating sequences, which contain no information, are lost. The lost repeats are then added back to the chromosome ends.
The discovery that the ends of chromosomes are made up of sequences repeated in tandem was made in 1978 by Elizabeth Blackburn and Joe Gall, who were studying the DNA in the unusual macronucleus of the single-celled ciliate Tetrahymena. Like other ciliates, Tetrahymena has a conventional micronucleus and an unusual macronucleus in which the chromosomes are fragmented into thousands of gene-size pieces with new ends added to each piece. With so many chromosome ends, Tetrahymena has about 40,000 telomeres and, as such, was the perfect choice to determine telomere composition. Blackburn and Gall were able to isolate the fragments containing the genes for ribosomal RNA (fragments called rDNA; see Chapter 9 for more on ribosomes) by using CsCl gradient centrifugation, the technique developed by Meselson and Stahl to isolate newly replicated E. coli DNA. The ends of rDNA fragments contained tandem arrays of the sequence TTGGGG. We now know that virtually all eukaryotes have short tandem repeats at their chromosome ends; however, the sequence is not exactly the same. Human chromosomes, for example, end in about 10 to 15 kb of tandem repeats of the sequence TTAGGG.
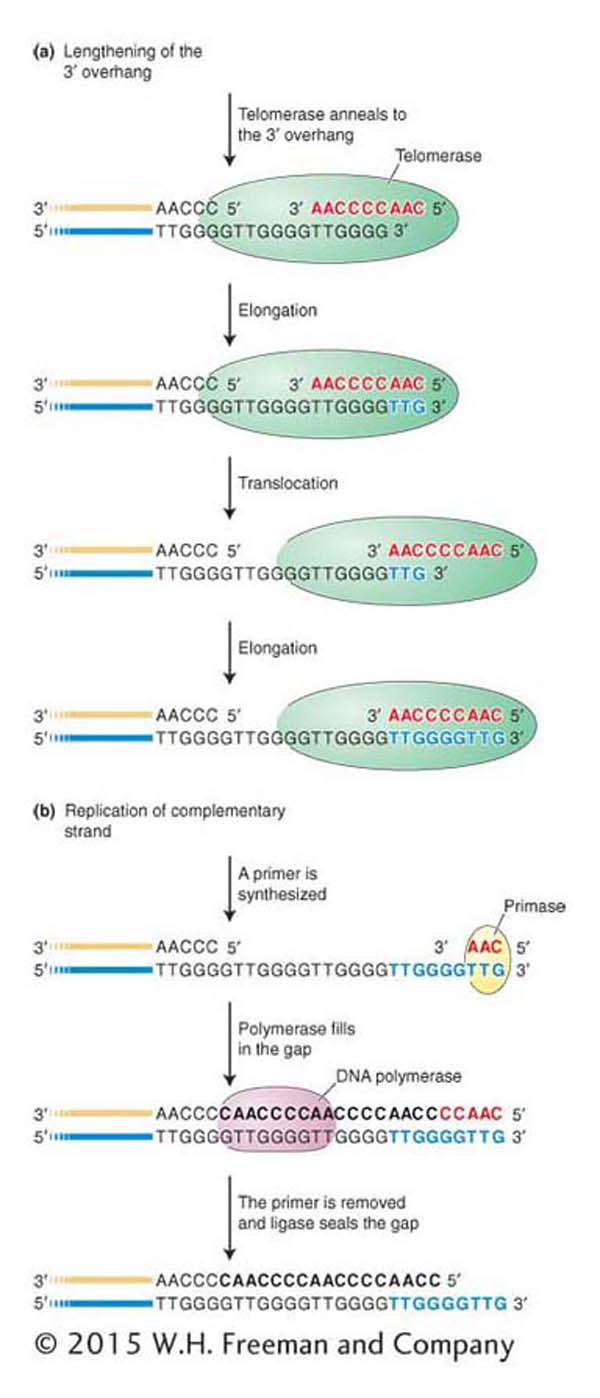
Figure 7-27: Telomere lengthening
Figure 7-27: Telomerase carries a short RNA molecule (red letters) that acts as a template for the addition of a complementary DNA sequence, which is added to the 3′ overhang (blue letters). To add another repeat, the telomerase translocates to the end of the repeat that it just added. The extended 3′ overhang can then serve as template for conventional DNA replication.
The question of how these repeats are actually added to chromosome ends after each round of replication was addressed by Elizabeth Blackburn and Carol Grieder. They hypothesized that an enzyme catalyzed the process. Working again with extracts from the Tetrahymena macronucleus, they identified an enzyme, which they called telomerase, that adds the short repeats to the 3′ ends of DNA molecules. Interestingly, the telomerase protein carries a small RNA molecule, part of which acts as a template for the synthesis of the telomeric repeat unit. In all vertebrates, including humans, the RNA sequence 3′-AAUCCC-5′ acts as the template for the 5′-TTAGGG-3′ repeat unit by a mechanism shown in Figure 7-27. Briefly, the telomerase RNA first anneals to the 3′ DNA overhang, which is then extended with the use of the telomerase’s two components: the small RNA (as template) and the protein (as polymerase activity). After the addition of a few nucleotides to the 3′ overhang, the telomerase RNA moves along the DNA so that the 3′ end can be further extended by its polymerase activity. The 3′ end continues to be extended by repeated movement of the telomerase RNA. Primase and DNA polymerase then use the very long 3′ overhang as a template to fill in the end of the other DNA strand. Working with Elizabeth Blackburn, a third researcher, Jack Szostak, went on to show that telomeres also exist in the less unusual eukaryote yeast. For contributing to the discovery of how telomeres protect chromosomes from shortening, Blackburn, Grieder, and Szostak were awarded the 2009 Nobel Prize in Medicine or Physiology.
One notable feature of this reaction is that RNA is serving as the template for the synthesis of DNA. As you saw in Chapter 1 (and will revisit in Chapter 8), DNA normally serves as the template of RNA synthesis in the process called transcription. It is for this reason that the polymerase of telomerase is said to have reverse transcriptase activity. We will revisit reverse transcriptase in Chapters 10 and 15.
In addition to preventing the erosion of genetic material after each round of replication, telomeres preserve chromosomal integrity by associating with proteins to form protective caps. These caps sequester the 3′ single-stranded overhang, which can be as much as 100 nucleotides long (Figure 7-28). Without this protective cap, the double-stranded ends of chromosomes would be mistaken for double-stranded breaks by the cell and dealt with accordingly. As you will see later, in Chapter 16, double-stranded breaks are potentially very dangerous because they can result in chromosomal instability that can lead to cancer and a variety of phenotypes associated with aging. For this reason, when a double-stranded break is detected, the cell responds in a variety of ways, depending, in part, on the cell type and the extent of the damage. For example, the double-stranded break can be fused to another break or the cell can limit the damage to the organism by stopping further cell division (called senescence) or by initiating a cell-death pathway (called apoptosis).
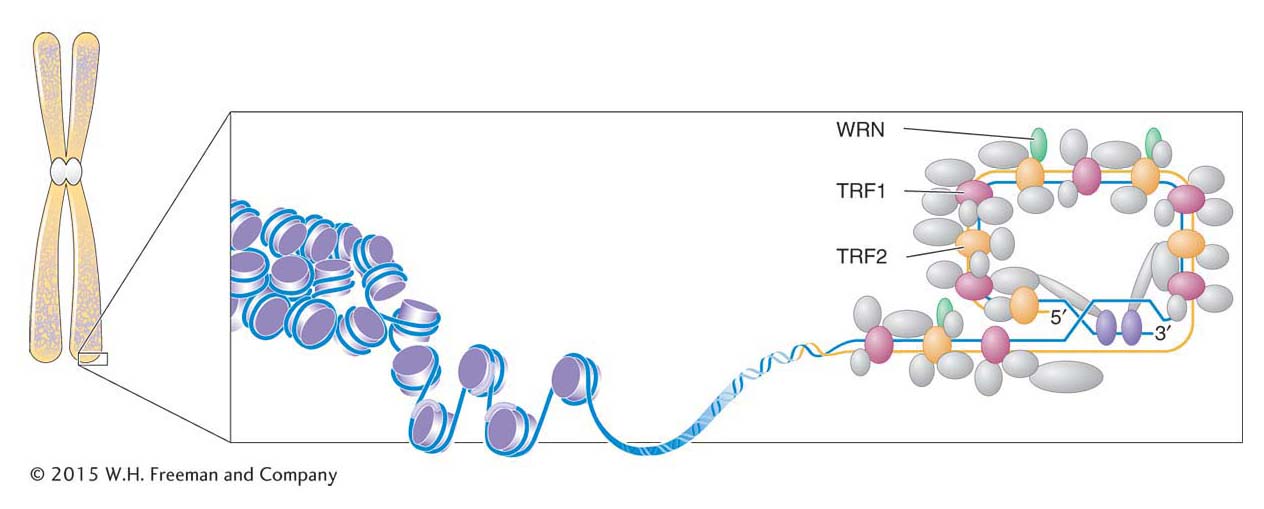
Figure 7-28: The telomeric cap structure
Figure 7-28: A “cap” protects the telomere at the end of a chromosome. The 3′ overhang is “hidden” when it displaces a DNA strand in a region where the telomeric repeats are double stranded. The proteins TRF1 and TRF2 bind to the telomeric repeats, and other proteins, including WRN, bind to TRF1 and TRF2, thus forming the protective telomeric cap.
KEY CONCEPT
Telomeres are specialized structures at the ends of chromosomes that contain tandem repeats of a short DNA sequence that is added to the 3′ end by the enzyme telomerase. Telomeres stabilize chromosomes by preventing the loss of genomic information after each round of DNA replication and by associating with proteins to form a cap that “hides” the chromosome ends from the cell’s DNA-repair machinery.
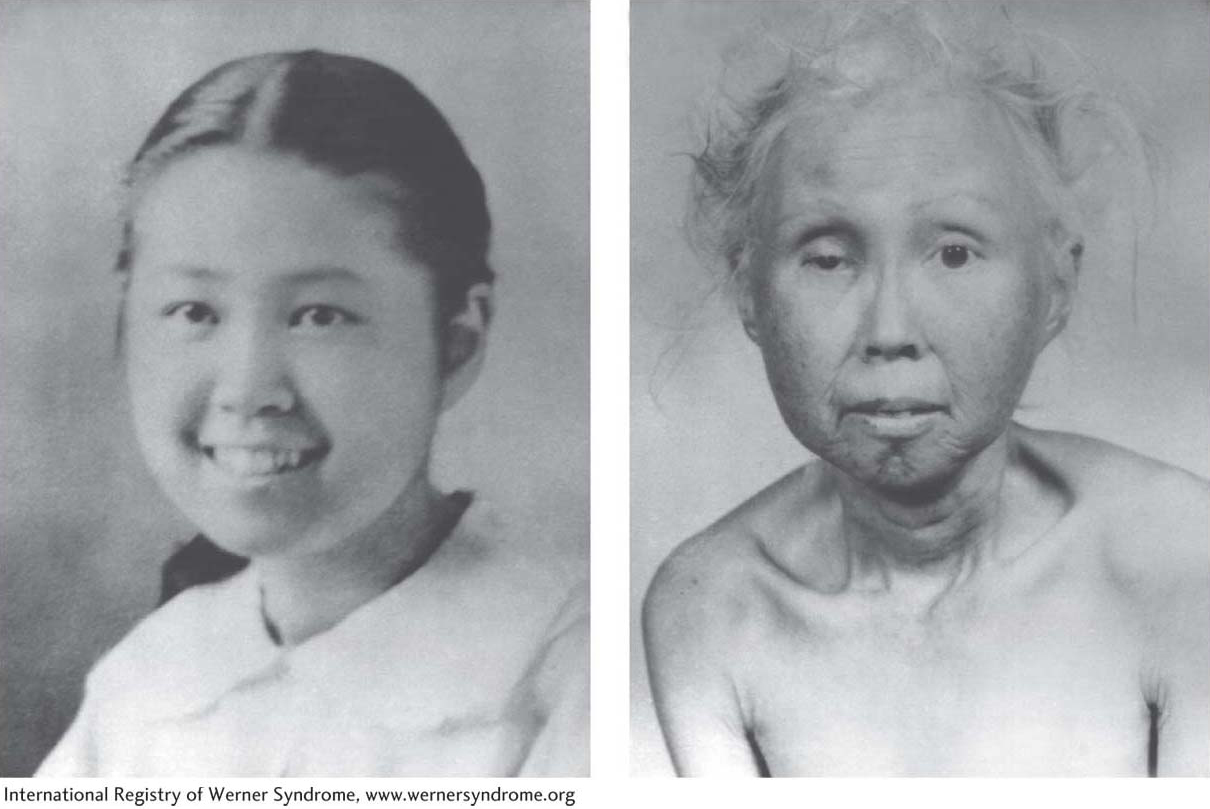
Figure 7-29: Werner syndrome causes premature aging
Figure 7-29: A woman with Werner syndrome at ages 15 and 48.
[International Registry of Werner Syndrome, www.wernersyndrome.org]
Surprisingly, although most germ cells have ample telomerase, somatic cells produce very little or no telomerase. For this reason, the chromosomes of proliferating somatic cells get progressively shorter with each cell division until the cell stops all divisions and enters a senescence phase. This observation led many investigators to suspect that there was a link between telomere shortening and aging. Geneticists studying human diseases that lead to a premature-aging phenotype have recently uncovered evidence that supports such a connection. People with Werner syndrome experience the early onset of many age-related events, including wrinkling of the skin, cataracts, osteoporosis, graying of the hair, and cardiovascular disease (Figure 7-29). Genetic and biochemical studies have found that afflicted people have shorter telomeres than those of normal people owing to a mutation in a gene called WRN, which encodes a protein (a helicase) that associates with proteins that comprise the telomere cap (TRF2, see Figure 7-28). This mutation is hypothesized to disrupt the normal telomere, resulting in chromosomal instability and the premature-aging phenotype. Patients with another premature-aging syndrome called dyskeratosis congenita also have shorter telomeres than those of healthy people of the same age, and they, too, harbor mutations in genes required for telomerase activity.
Geneticists are also very interested in connections between telomeres and cancer. Unlike normal somatic cells, most cancerous cells have telomerase activity. The ability to maintain functional telomeres may be one reason that cancer cells, but not normal cells, can grow in cell culture for decades and are considered to be immortal. As such, many pharmaceutical companies are seeking to capitalize on this difference between cancerous and normal cells by developing drugs that selectively target cancer cells by inhibiting telomerase activity.



