Small nuclear RNAs (snRNAs): the mechanism of exon splicing
After the discovery of exons and introns, scientists turned their attention to the mechanism of RNA splicing. Because introns must be precisely removed and exons precisely joined, the first approach was to compare the sequences of pre-mRNAs for clues to how introns and exons are recognized. Figure 8-15 shows the exon–intron junctions of pre-mRNAs. These junctions are the sites at which the splicing reactions take place. At these junctions, certain specific nucleotides were found to be nearly identical across genes and across species; they have been highly conserved because they participate in the splicing reactions. Each intron is cut at each end, and these intron ends almost always have GU at the 5′ end and AG at the 3′ end (the GU-AG rule). Another invariant site is an A residue (the branch point A) between 15 and 45 nucleotides upstream of the 3′ splice site. The nucleotides flanking the highly conserved ones also are conserved, but to a lesser degree. The existence of conserved nucleotide sequences at splice junctions suggested that there must be cellular machinery that recognizes these sequences and carries out splicing. As is often the case in scientific research, the splicing machinery was found by accident and the mechanism of splicing was entirely unexpected.
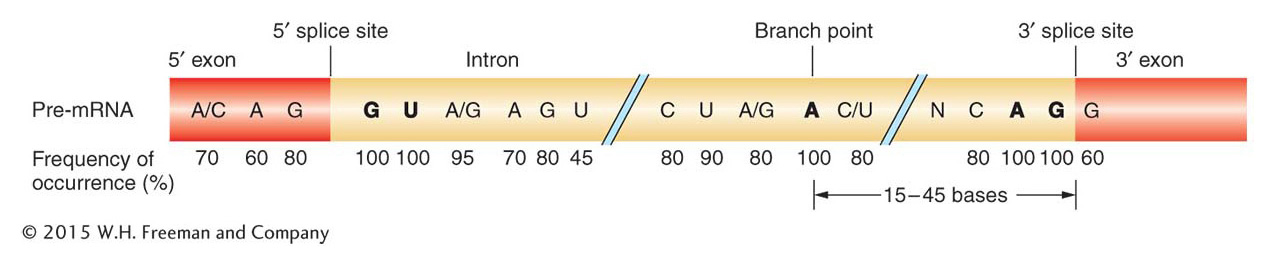
Figure 8-15: Conserved sequences related to intron splicing
Figure 8-15: Conserved nucleotide sequences are present at the junctions of introns and exons. The numbers below the nucleotides indicate the percentage of similarity among organisms. Of particular importance are the G and U residues at the 5′ end, the A and G residues at the 3′ end, and the A residue labeled “branch point” (see Figure 8-17 for a view of the branch structure). N represents any base.
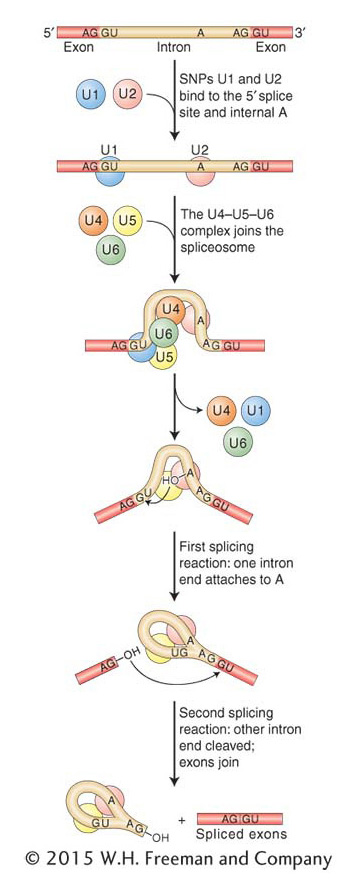
Figure 8-16: Spliceosome assembly and function
Figure 8-16: The spliceosome is composed of several snRNPs that attach sequentially to the RNA, taking up positions roughly as shown. Alignment of the snRNPs results from hydrogen bonding of their snRNA molecules to the complementary sequences of the intron. In this way, the reactants are properly aligned and the two splicing reactions can take place. The chemistry of these reactions can be seen in more detail in Figure 8-17.
A serendipitous finding in the laboratory of Joan Steitz led to the discovery of components of the splicing machinery. Patients with a variety of autoimmune diseases, including systemic lupus erythematosis, produce antibodies against their own proteins. In the course of analyzing blood samples from patients with lupus, Steitz and colleagues identified antibodies that could bind to a large molecular complex of small RNAs and proteins. Because this riboprotein complex was localized in the nucleus, the RNA components were named small nuclear RNAs. The snRNAs were found to be complementary to the consensus sequences at splice junctions, leading scientists to hypothesize a role for the snRNAs in the splicing reaction. The conserved nucleotides in the transcript are now known to be recognized by five small nuclear ribonucleoproteins (snRNPs), which are complexes of protein and one of five snRNAs (U1, U2, U4, U5, and U6). These snRNPs and more than 100 additional proteins are part of the spliceosome, the large biological machine that removes introns and joins exons. Components of the spliceosome interact with the CTD, as suggested in Figure 8-13b.
These components of the spliceosome attach to intron and exon sequences, as shown in Figure 8-16. The U1 and U2 snRNPs help to align the splice sites at either end of an intron by forming hydrogen bonds to the conserved intron and exon sequences. Then the snRNPs recruit U4, U5, and U6 to and form the spliceosome, which catalyzes the removal of the intron through two consecutive splicing steps (see Figure 8-16). The first step attaches one end of the intron to the conserved internal adenine, forming a loop structure, the shape of a cowboy’s lariat. The second step releases the lariat and joins the two adjacent exons. Figure 8-17 portrays the chemistry behind intron removal and exon splicing. Chemically, the two steps are transesterification reactions between the conserved nucleotides. Hydroxyl groups at the 2′ and 3′ positions of ribonucleotides are key reaction participants.
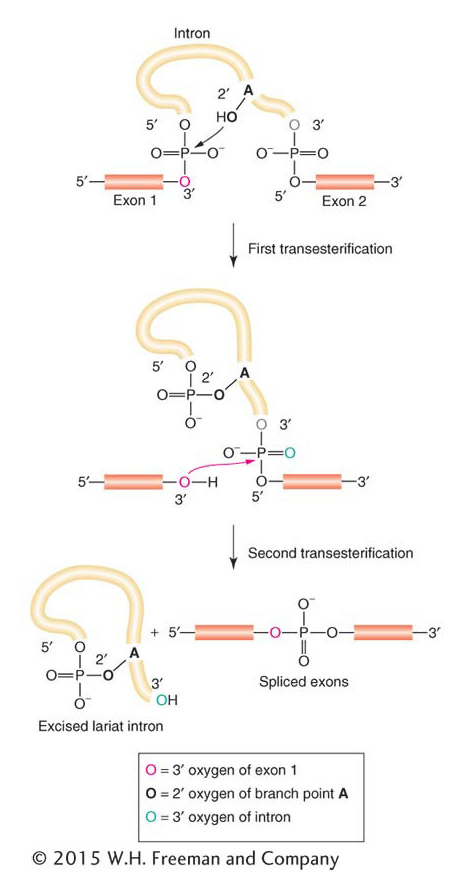
Figure 8-17: Reactions in exon splicing
Figure 8-17: Two transesterification reactions take place in the splicing of RNA: first, to join the 5′ donor end of the intron to the internal branch point (first reaction in Figure 8-16) and, second, to join the two exons together (second reaction in Figure 8-16).
Self-splicing introns and the RNA world
Two exceptional cases of RNA processing led to a discovery considered by some to be as important as that of the double-helical structure of DNA. In 1981, Tom Cech and co-workers reported that, in a test tube, the primary transcript of an rRNA from the ciliate protozoan Tetrahymena could excise a 413-nucleotide intron from itself without the addition of any protein (Figure 8-18). Subsequently, other introns have been shown to have this property and have come to be known as self-splicing introns. A few years earlier while studying the processing of tRNA in bacteria, Sidney Altman identified a ribonucleoprotein (called RNase P) responsible for cutting the pre-tRNA molecule at a specific site. The big surprise came when they determined that the catalytic activity of RNase P resided in the RNA component of the enzyme rather than in the protein component. Cech and Altman’s findings are considered landmark discoveries because they marked the first time that biological molecules other than protein were shown to catalyze reactions. As such, it was fitting that they received the Nobel Prize in Chemistry in 1989.
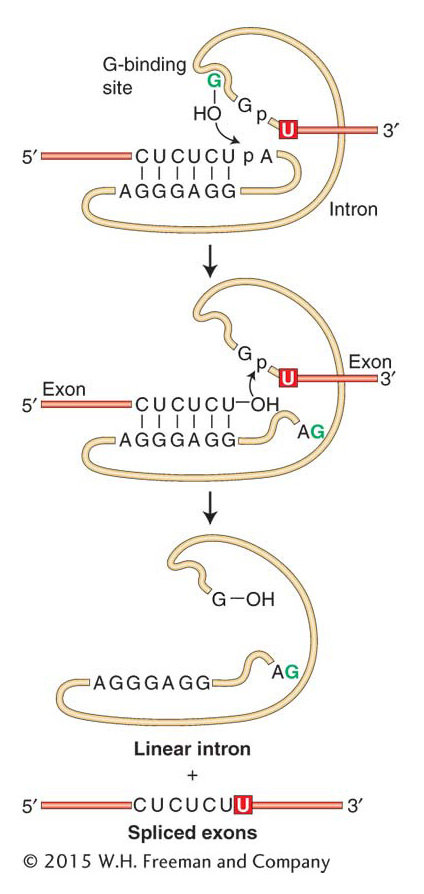
Figure 8-18: Self-splicing reaction
Figure 8-18: The self-splicing intron from Tetrahymena executes two transesterification reactions to excise itself from RNA.
The discovery of self-splicing introns has led to a reexamination of the role of the snRNAs in the spliceosome. The most recent studies indicate that intron removal is catalyzed by the snRNAs and not by the protein component of the spliceosome. As you will see in Chapter 9, the RNAs in the ribosome (the rRNAs), not the ribosomal proteins, have the central role in most of the important events of protein synthesis. The numerous examples of ribozymes have provided solid evidence for a theory called the RNA world, which holds that RNA must have been the genetic material in the first cells because only RNA is known to both encode genetic information and catalyze biological reactions.
KEY CONCEPT
Intron removal and exon joining are catalyzed by RNA molecules. In eukaryotes, the snRNAs of the spliceosome catalyze the removal of introns from pre-mRNA. Some introns are self-splicing; in these cases, the intron itself catalyzes its own removal. RNAs capable of catalysis are called ribozymes.



