miRNAs are important regulators of gene expression
The first of this type of small RNA was discovered in 1993 by Victor Ambros and colleagues while they were studying the lin-4 gene of the roundworm C. elegans. Because mutations in lin-4 resulted in abnormal larvae, it was hypothesized that this gene encoded a protein that was required for normal larval development. Thus, it came as a surprise when the group isolated the lin-4 gene and reported that, rather than encode a protein, it produced two small RNAs of 22 nucleotides and 61 nucleotides. They then found that the 22-nucleotide RNA was produced by processing the larger 61-nucleotide RNA. Finally, they found that the 22-nucleotide RNA repressed the expression of certain other genes by base pairing with their mRNAs.
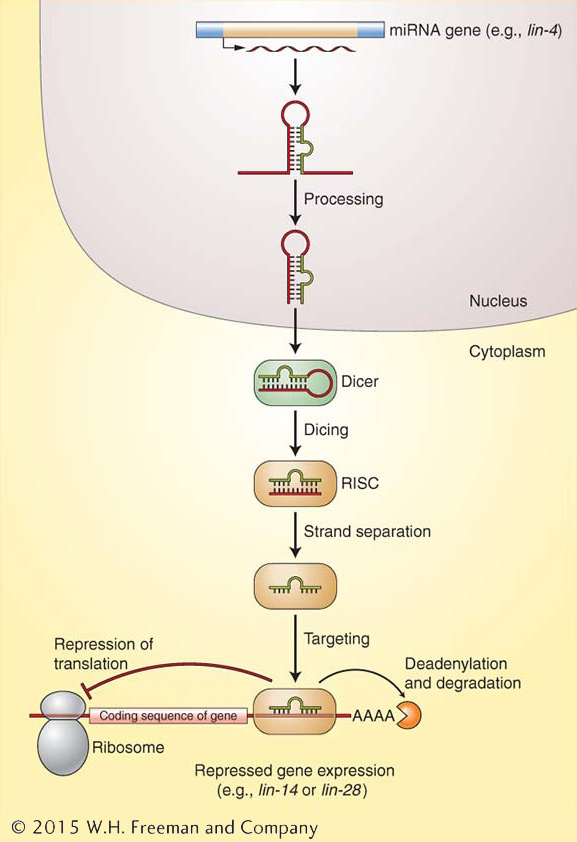
Figure 8-19: miRNAs halt translation from targeted genes
Figure 8-19: miRNAs are synthesized by pol II as longer RNAs that are processed in several steps to their mature form. Once fully processed, miRNAs bind to RISC and direct its activities to reduce the expression of complementary mRNAs by either repressing their translation or promoting their degradation.
The 22-nucleotide RNA product of the lin-4 gene was the first member to be discovered of a very large class of RNAs called microRNAs (miRNAs), now known to be present in the genomes of plants and animals. Most miRNAs act to repress the expression of genes. In fact, it is estimated that plant and animal genomes each have up to a thousand miRNAs that in turn regulate the expression of thousands of genes. Like the product of lin-4, many miRNAs are initially transcribed by RNA polymerase II as a longer RNA from a gene that produces only an RNA product. The longer RNA assumes a double-stranded stem-loop structure with a mismatched base in the stem (Figure 8-19). The RNA is processed in the nucleus to a smaller but not yet final form, then exported to the cytoplasm. There, two biological machines, both with the ability to cleave RNA, take part in a two-step process. One machine, called Dicer, recognizes double-stranded RNA (dsRNA) molecules and cleaves them into ~22-nucleotide products. A second machine, called RISC (RNA-induced silencing complex), binds to the short dsRNA and unwinds it into the biologically active single-stranded miRNA. The miRNA, still bound to RISC, binds to complementary mRNAs. RISC then represses the translation of these mRNAs into protein or removes the poly(A) tail, which hastens mRNA degradation. In the example shown in Figure 8-19, the lin-4 miRNA binds to lin-14 and lin-28 mRNAs and represses their translation.
You will learn more about the function of miRNAs in Chapters 12 and 13. The key point to remember is that part of an miRNA is complementary to the RNA of the gene it regulates. When the regulated gene needs to be shut down or its expression reduced, the miRNA gene is transcribed into RNA and that RNA binds to the RNA of the regulated gene, interfering with translation into protein or promoting its degradation.
KEY CONCEPT
miRNAs are processed from longer RNA pol II transcripts by Dicer, which binds to double-stranded RNAs. The biologically active single-stranded miRNA binds to RISC and guides it to complementary sequences in protein-coding mRNAs, where RISC either represses translation or promotes mRNA degradation.
siRNAs ensure genome stability
Scientists soon found a different case of dsRNA that could repress gene expression prior to translation. This finding led to the discovery of a second type of short RNA, siRNAs. This second type of short RNA has a very different origin and function from miRNAs. In contrast to miRNAs, an siRNA silences the gene that produces it. Thus, it is not used to regulate other genes, but rather to shut off undesirable genetic elements that insert into the genome. Such undesirable elements could be the genes in an infecting virus, or they could be internal genetic elements called transposons that you will learn about in Chapter 15.
In 1998, five years after the discovery of miRNAs, Andrew Fire and Craig Mello reported that they had found a potent way to selectively turn off genes, also in the roundworm C. elegans. Fire and Mello discovered that, by injecting dsRNA copies of a C. elegans gene into C. elegans embryos, they were able to block the synthesis of the protein product of that gene (Figure 8-20a). The selective shutting off of the gene by this procedure is called gene silencing. The dsRNA had been synthesized in the laboratory and was composed of a sense (coding) RNA strand and a complementary antisense RNA strand. In their initial experiment, Fire and Mello injected dsRNA copies of the unc-22 gene into C. elegans embryos and watched as the embryos grew into adults that twitched and had muscle defects. This result was exciting because unc-22 was known to encode a muscle protein and null mutants of unc-22 displayed the same twitching and muscle defects. Taken together, these observations indicated that the injected dsRNA prevented the production of the Unc-22 protein. For their discovery of a new way to silence genes, Fire and Mello were awarded the Nobel Prize in Medicine or Physiology in 2004.
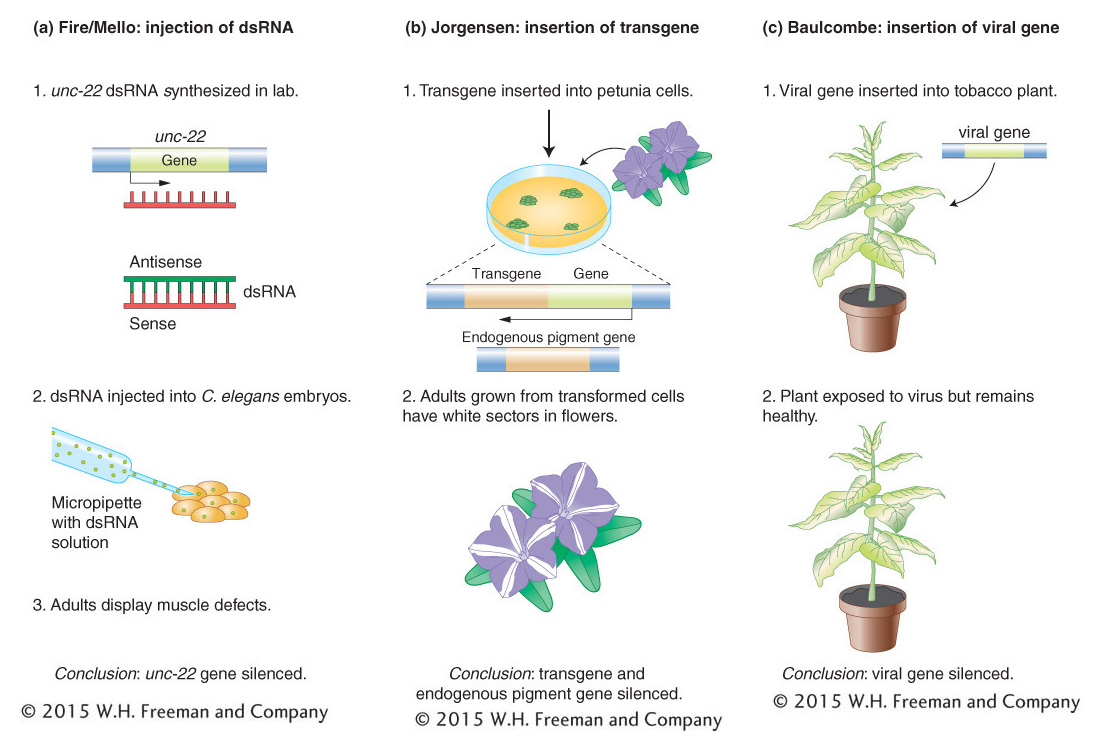
Figure 8-20: Three experiments demonstrating gene silencing
Figure 8-20: Three experiments reveal key features of gene silencing. (a) Fire and Mello demonstrated that dsRNA copies can selectively silence genes in C. elegans. (b) Jorgensen discovered that a transgene can silence an endogenous petunia gene necessary for floral color. (c) Baulcombe showed that plants with a copy of a viral transgene were resistant to viral infection and produced siRNAs complementary to the viral genome.
If instead of a dsRNA copy of a gene, what would happen if a DNA copy of a gene normally found in an organism were inserted into its genome? In such an experiment the introduced gene would be an example of a transgene, which is short for “transformed gene.” A transgene is a gene that has been introduced into the chromosomes of an organism in the laboratory. An organism containing a transgene in its genome is called either a transgenic organism or genetically modified organism (or the popular abbreviated term GMO). This experiment was actually done in 1990 by Rich Jorgensen, a plant scientist studying the color of flowers in petunias.
One of the greatest joys of doing scientific research is observing a completely unexpected result. This is precisely what happened to Jorgensen after he inserted a petunia gene that encodes an enzyme necessary for the synthesis of purple-blue floral pigment into a normal petunia plant having purple-blue flowers (Figure 8-20b). He expected that the floral color of this transgenic plant would be unchanged. After all, the transgenic plant had two good genes necessary for pigment production— one at its usual locus in the petunia genome (called the endogenous gene; in Figure 8-20b it is called the pigment gene) plus the introduced transgene that was inserted elsewhere in the genome. However, instead of purple flowers, the transgenic plants displayed the unusual floral patterns shown in Figure 8-21. In a totally unexpected outcome, the transgene triggered suppression of both the transgene and the endogenous pigment gene, resulting in white flowers or, more commonly, white floral sectors. This phenomenon is called cosuppression because the expression of both the introduced transgene and the endogenous copy is suppressed.
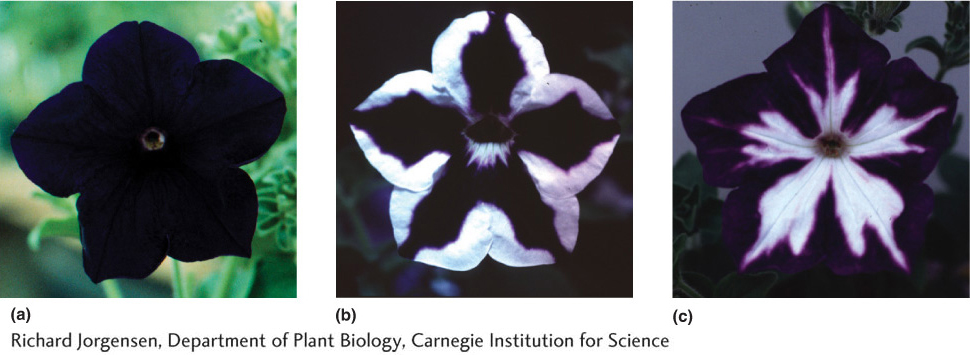
Figure 8-21: Petunia flowers demonstrating cosuppression
Figure 8-21: (a) The wild-type (no transgene) phenotype. (b and c) So-called cosuppression phenotypes resulting from the transformation of the wild-type petunia shown in part a with a petunia gene required for pigmentation. In the colorless regions, both the transgene and the chromosomal copy of the same gene have been inactivated.
[Richard Jorgensen, Department of Plant Biology, Carnegie Institution for Science]
To review, introduction of either a dsRNA copy of a gene or the gene itself into an organism can silence that gene. To understand why these different experiments led to the same result, scientists hypothesized that the insertion of the transgene led to the synthesis of antisense RNA, which could complement with sense RNA to produce dsRNA. Because scientists cannot control where transgenes insert, some transgenes will end up next to genes in an opposite orientation (Figure 8-22). Transcription initiated at the gene promoter can “read through” into the transgene and produce a very long “chimeric” RNA containing both the sense strand of the gene and the antisense strand of the transgene. Double-stranded RNA will then form when the antisense part of the long RNA hybridizes with sense RNA produced by either the transgene or the endogenous gene.
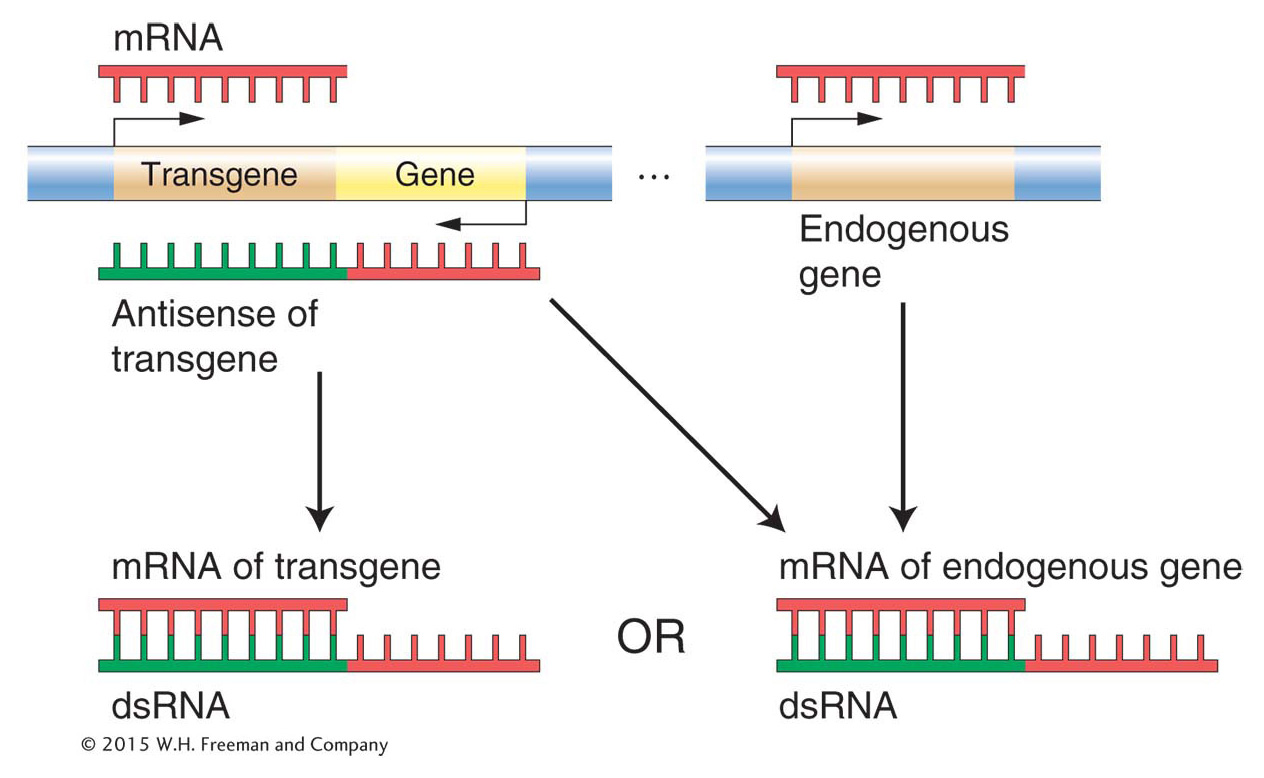
Figure 8-22: Two ways to generate double-stranded RNA from a transgene
Figure 8-22: The insertion of a transgene can lead to the production of double-stranded RNA (dsRNA) if the transgene is inserted at the end of a gene in the opposite orientation. The antisense RNA produced when the neighboring gene is transcribed can bind to the mRNA of either the transgene itself or the endogenous gene to produce dsRNA.
Thus, dsRNA is a common feature of this form of gene silencing. However, the function of this process is clearly not to shut off genes introduced by scientists. What is the normal role of this form of gene silencing in the cell? An important clue came from the experiments conducted by another plant scientist, David Baulcombe, who was investigating the reason why tobacco plants that were engineered to express a viral gene were resistant to subsequent infection by the virus. In this experiment the viral gene is another example of a transgene, which was, in this case, introduced into the tobacco genome (see Figure 8-20c). A key difference between this and the petunia experiment was that tobacco plants do not normally have a viral gene in their genome. So this experiment suggested that this form of gene silencing functions to silence invading viruses.
Baulcombe and his co-workers found that the resistant plants, and only the resistant plants, produced large amounts of short RNAs, 25 nucleotides in length, that were complementary to the viral genome. Significantly, short RNAs related to the endogenous genes have also been found to be present during gene silencing in the worm and in the petunia. The short RNAs generated during viral resistance and gene silencing associated with either injected dsRNAs or transgenes are now collectively called small interfering RNAs (siRNAs). The phenomena that results in gene silencing and viral resistance through the production of siRNAs is called RNA interference (RNAi). The short RNAs (21-31 nucleotides in length) are now classified as one of three types depending on their biogenesis: miRNAs or siRNAs (both 21-25 nucleotides) or the recently discovered piwi-interacting RNAs (piRNAs, 24-31 nucleotides). Because the mechanism of piRNA synthesis is still under investigation, we will focus on the better characterized miRNAs and siRNAs.
Similar mechanisms generate siRNA and miRNA
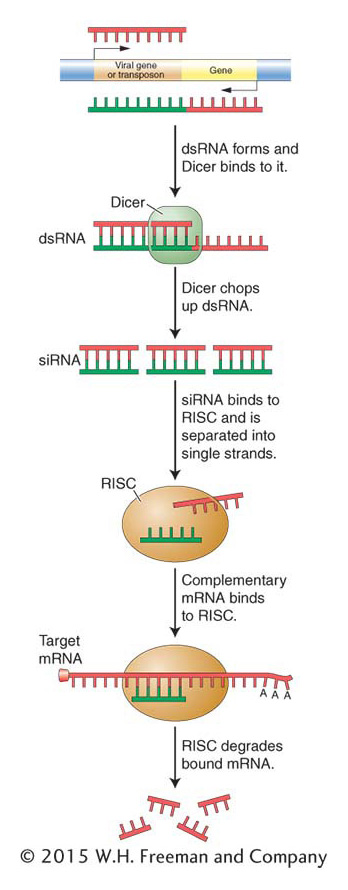
Figure 8-23: siRNAs degrade mRNA from viral genes or transposons
Figure 8-23: In the RNA interference pathway, double-stranded RNA (dsRNA) specifically interacts with the Dicer complex, which chops the dsRNA up. The RNA-induced silencing complex (RISC) uses the small dsRNAs to find and destroy homologous mRNA transcribed from the target DNA, thereby repressing gene expression.
As we have seen, siRNAs can arise from an antisense copy of any source of mRNA in the genome: from endogenous genes to transgenes to invading viruses. However, the most likely source of antisense RNA is not an organism’s own genes, but rather foreign DNA that inserts into the genome. In this regard, it would be correct to think of siRNAs as the product of a genome immune system that detects the insertion of foreign DNA by, in some cases, promoting the synthesis of antisense mRNA. Complementarity between sense and antisense RNAs produces dsRNAs, which, as in the miRNA pathway, are recognized by Dicer and cleaved into short double-stranded products that are bound by RISC (Figure 8-23). As with miRNAs, RISC unwinds the product into the biologically active single-stranded siRNA that targets RISC to complementary mRNAs so they can be degraded. Unlike miRNAs, complementarity between siRNAs and mRNAs is perfect; there are no mismatches. This is because of their different origins: siRNAs are derived from the same gene, whereas miRNAs come from a different gene. This difference is probably responsible for the different outcomes: miRNAs direct RISC to repress the translation of an mRNA or degrade mRNAs when they are being translated, whereas siRNAs direct RISC to degrade the mRNA directly.
As discussed above, the production of siRNAs probably plays an important role in viral defense. However, its most important role may be to protect the hereditary material of an organism from genetic elements in its own genome. In Chapter 15, you will learn about the transposable elements that constitute a huge fraction of the genomes of multicellular eukaryotes, including humans. These elements can amplify themselves and move to new locations, creating an obvious threat to the integrity of the genome. Just like the introduction of transgenes by scientists, the movement of transposable elements into new chromosomal locations can trigger the production of siRNAs by generating dsRNA. The siRNAs eventually inactivate the transposable elements in part by preventing the production of the protein products needed for their movement and amplification.
KEY CONCEPT
Antisense RNA is frequently formed in response to the insertion of foreign DNA into the genome. Dicer detects double-stranded RNA that forms between antisense and sense RNA and processes it into short RNAs. RISC binds a short RNA and unwinds it to form biologically active siRNA. The siRNA targets RISC to a perfectly complementary mRNA, which is degraded, thus silencing the expression of the foreign DNA.




