9.3 tRNA: The Adapter
Once the genetic code was deciphered, scientists began to wonder how the sequence of amino acids of a protein was determined by the triplet codons of the mRNA. An early model, quickly dismissed as naive and unlikely, proposed that the mRNA codons could fold up and form 20 distinct cavities that directly bind specific amino acids in the correct order. Instead, in 1958, Crick recognized the following:
It is therefore a natural hypothesis that the amino acid is carried to the template by an adapter molecule, and that the adapter is the part which actually fits on to the RNA. In its simplest form [this hypothesis] would require twenty adapters, one for each amino acid.1
330
He speculated that the adapter “might contain nucleotides. This would enable them to join on the RNA template by the same ‘pairing’ of bases as is found in DNA.” Furthermore, “a separate enzyme would be required to join each adapter to its own amino acid.”
We now know that Crick’s “adapter hypothesis” is largely correct. Amino acids are in fact attached to an adapter (recall that adapters constitute a special class of stable RNAs called transfer RNAs). Each amino acid becomes attached to a specific tRNA, which then brings that amino acid to the ribosome, the molecular complex that will attach the amino acid to a growing polypeptide.
Codon translation by tRNA
The structure of tRNA holds the secret of the specificity between an mRNA codon and the amino acid that it designates. The single-
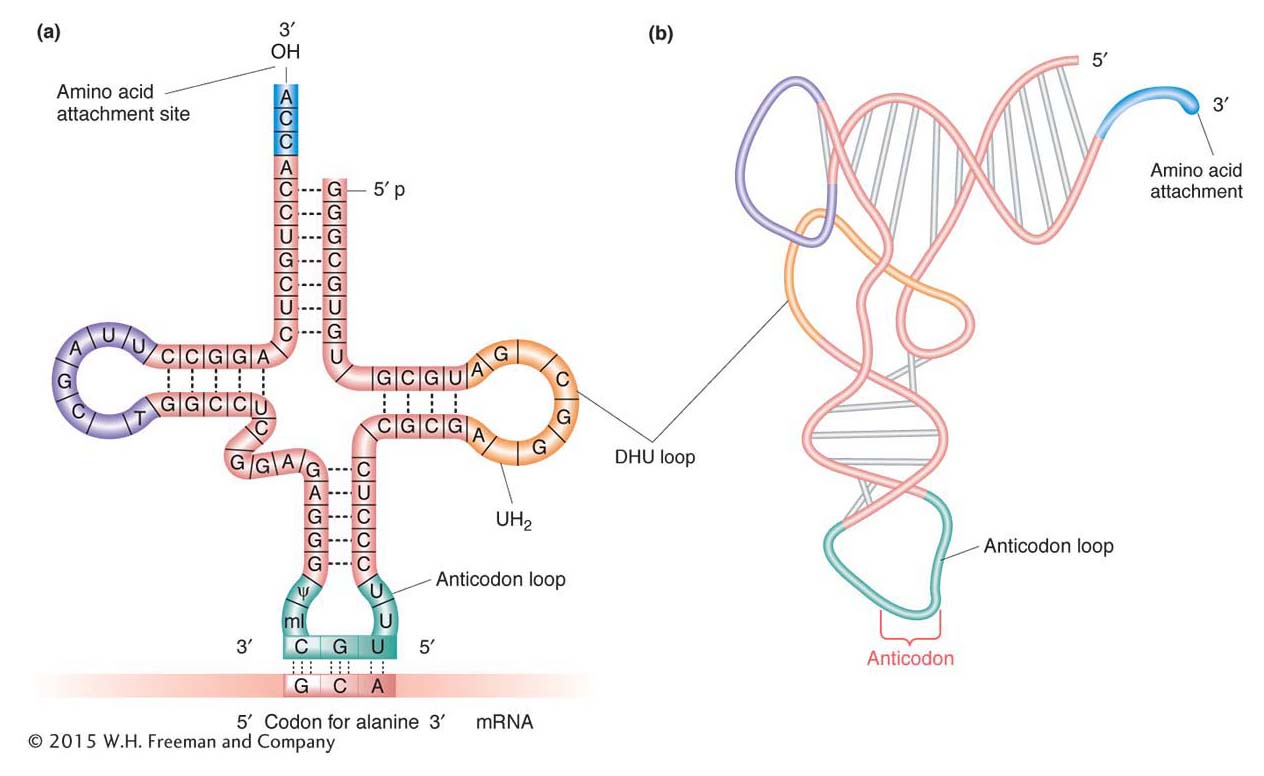
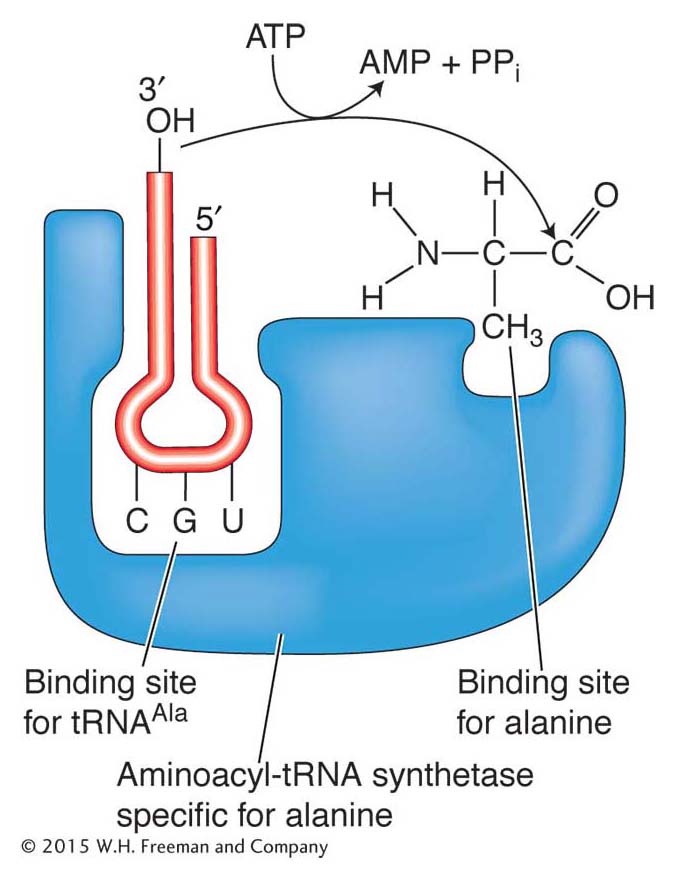
Amino acids are attached to tRNAs by enzymes called aminoacyl-
331
A tRNA normally exists as an L-
What would happen if the wrong amino acid were covalently attached to a tRNA? A convincing experiment answered this question. The experiment used cysteinyl-
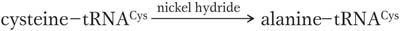
Protein synthesized with this hybrid species had alanine wherever we would expect cysteine. The experiment demonstrated that the amino acids are “illiterate”; they are inserted at the proper position because the tRNA “adapters” recognize the mRNA codons and insert their attached amino acids appropriately. Thus, the attachment of the correct amino acid to its cognate tRNA is a critical step in ensuring that a protein is synthesized correctly. If the wrong amino acid is attached, there is no way to prevent it from being incorporated into a growing protein chain.
Degeneracy revisited
As can be seen in Figure 9-5, the number of codons for a single amino acid varies, ranging from one codon (UGG for tryptophan) to as many as six (UCC, UCU, UCA, UCG, AGC, or AGU for serine). Why the genetic code contains this variation is not exactly clear, but two facts account for it:
Most amino acids can be brought to the ribosome by several alternative tRNA types. Each type has a different anticodon that base-
pairs with a different codon in the mRNA. Certain charged tRNA species can bring their specific amino acids to any one of several codons. These tRNAs recognize and bind to several alternative codons, not just the one with a complementary sequence, through a loose kind of base pairing at the 3′ end of the codon and the 5′ end of the anticodon. This loose pairing is called wobble.
Wobble is a situation in which the third nucleotide of an anticodon (at the 5 ′ end) can form either of two alignments (Figure 9-9). This third nucleotide can form hydrogen bonds either with its normal complementary nucleotide in the third position of the codon or with a different nucleotide in that position.
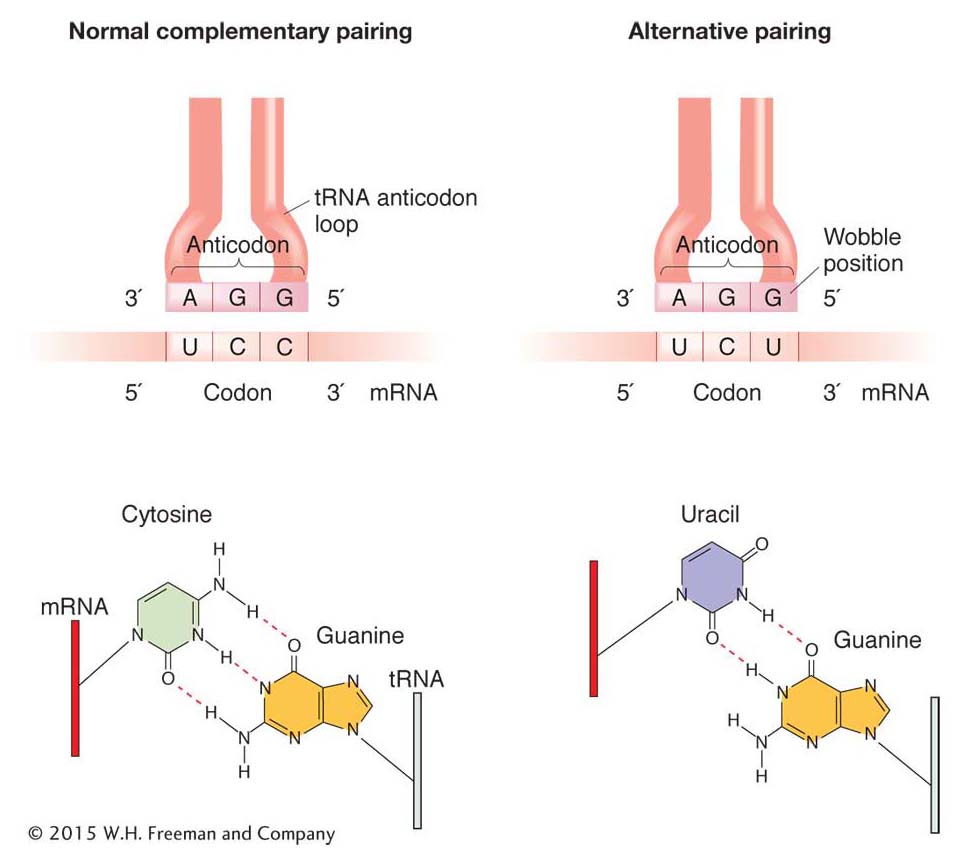
“Wobble rules” dictate which nucleotides can and cannot form hydrogen bonds with alternative nucleotides through wobble (Table 9-1). In Table 9-1, the letter I stands for inosine, one of the rare bases found in tRNA, often in the anticodon.
|
5′ end of anticodon |
3′ end of codon |
|---|---|
|
G |
C or U |
|
C |
G only |
|
A |
U only |
|
U |
A or G |
|
I |
U, C, or A |
332