Neurospora crassa
| Genetic "Vital Statistics" | |
|---|---|
|
Genome size: |
43 Mb |
|
Chromosomes: |
7 autosomes (n = 7) |
|
Number of genes: |
10,000 |
|
Percentage with human homologs: |
6% |
|
Average gene size: |
1.7 kb, 1.7 introns/gene |
|
Transposons: |
rare |
|
Genome sequenced in: |
2003 |
Neurospora crassa

Key organism for studying:
Genetics of metabolism and uptake
Genetics of crossing over and meiosis
Fungal cytogenetics
Polar growth
Circadian rhythms
Interactions between nucleus and mitochondria

Neurospora crassa, the orange bread mold, was one of the first eukaryotic microbes to be adopted by geneticists as a model organism. Like yeast, it was originally chosen because of its haploidy, its simple and rapid life cycle, and the ease with which it can be cultured. Of particular significance was the fact that it will grow on a medium with a defined set of nutrients, making it possible to study the genetic control of cellular chemistry. In nature, it is found in many parts of the world growing on dead vegetation. Because fire activates its dormant ascospores, it is most easily collected after burns—
Special features
Neurospora holds the speed record for fungi because each hypha grows more than 10 cm per day. This rapid growth, combined with its haploid life cycle and ability to grow on defined medium, has made it an organism of choice for studying biochemical genetics of nutrition and nutrient uptake.
Another unique feature of Neurospora (and related fungi) allows geneticists to trace the steps of single meioses. The four haploid products of one meiosis stay together in a sac called an ascus. Each of the four products of meiosis undergoes a further mitotic division, resulting in a linear octad of eight ascospores. This feature makes Neurospora an ideal system in which to study crossing over, gene conversion, chromosomal rearrangements, meiotic nondisjunction, and the genetic control of meiosis itself. Chromosomes, although small, are easily visible, and so meiotic processes can be studied at both the genetic and the chromosomal levels. Hence, in Neurospora, fundamental studies have been carried out on the mechanisms underlying these processes.
Life Cycle
N. crassa has a haploid eukaryotic life cycle. A haploid asexual spore (called a conidium) germinates to produce a germ tube that extends at its tip. Progressive tip growth and branching produce a mass of branched threads (called hyphae), which forms a compact colony on growth medium. Because hyphae have no cross walls, a colony is essentially one cell containing many haploid nuclei. The colony buds off millions of asexual spores, which can disperse in air and repeat the asexual cycle.
In N. crassa’s sexual cycle, there are two identical-
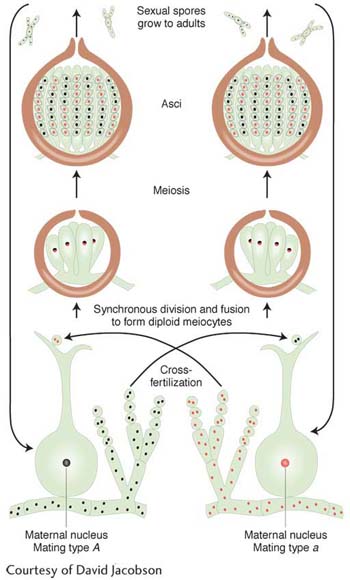
Length of life cycle: 4 weeks for sexual cycle
Genetic analysis
Genetic analysis is straightforward. Stock centers provide a wide range of mutants affecting all aspects of the biology of the fungus. Neurospora genes can be mapped easily by crossing them with a bank of strains with known mutant loci or known RFLP alleles. Strains of opposite mating type are crossed simply by growing them together. A geneticist with a handheld needle can pick out a single ascospore for study. Hence, analyses in which either complete asci or random ascospores are used are rapid and straightforward.
799
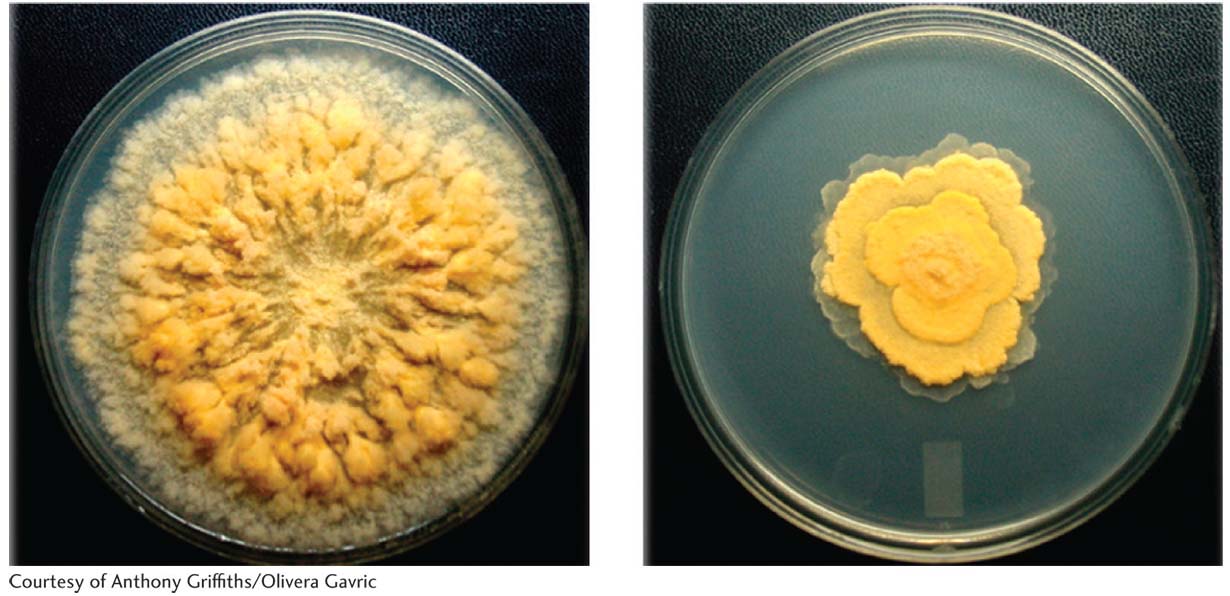
Because Neurospora is haploid, newly obtained mutant phenotypes are easily detected with the use of various types of screens and selections. A favorite system for study of the mechanism of mutation is the ad-
Although vegetative diploids of Neurospora are not readily obtainable, geneticists are able to create a “mimic diploid,” useful for complemenation tests and other analyses requiring the presence of two copies of a gene. Namely, the fusion of two different strains produces a heterokaryon, an individual containing two different nuclear types in a common cytoplasm. Heterokaryons also enable the use of a version of the specific-
Techniques of Genetic Manipulation
|
Standard mutagenesis: |
|
|
Chemicals and radiation |
Random somatic mutations |
|
Transposon mutagenesis |
Not available |
|
Transgenesis: |
|
|
Plasmid- |
Random insertion |
|
Targeted gene knockouts: |
|
|
RIP |
GC → AT mutations in transgenic duplicate segments before a cross |
|
Quelling |
Somatic posttranscriptional inactivation of transgenes |
Genetic engineering
Transgenesis. The first eukaryotic transformation was accomplished in Neurospora. Today, Neurospora is easily transformed with the use of bacterial plasmids carrying the desired transgene, plus a selectable marker such as hygromycin resistance to show that the plasmid has entered. No plasmids replicate in Neurospora, and so a transgene is inherited only if it integrates into a chromosome.
Targeted knockouts. In special strains of Neurospora, transgenes frequently integrate by homologous recombination. Hence, a trans-
Main contributions
George Beadle and Edward Tatum used Neurospora as the model organism in their pioneering studies on gene–
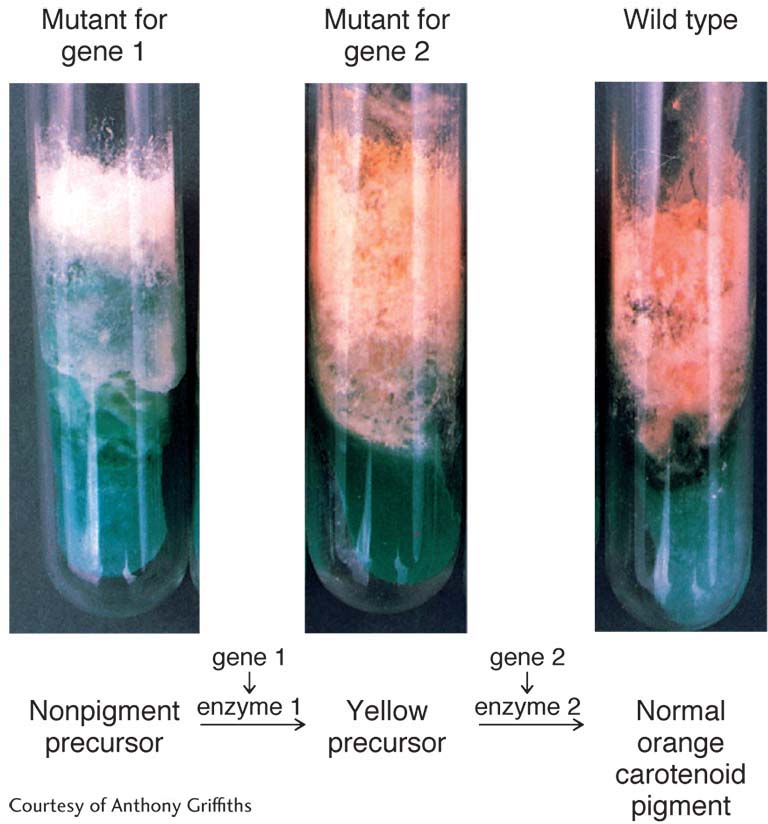
Pioneering work has been done on the genetics of meiotic processes, such as crossing over and disjunction, and on co-
Neurospora serves as a model for the multitude of pathogenic filamentous fungi affecting crops and humans because these fungi are often difficult to culture and manipulate genetically. It is even used as a simple eukaryotic test system for mutagenic and carcinogenic chemicals in the human environment.
Because crosses can be made by using one parent as female, the cycle is convenient for the study of mitochondrial genetics and nucleus–
Other areas of contribution
Fungal diversity and adaptation
Cytogenetics (chromosomal basis of genetics)
Mating-
type genes Heterokaryon-
compatibility genes (a model for the genetics of self and nonself recognition)
800