Drosophila melanogaster
| Genetic "Vital Statistics" | |
|---|---|
|
Genome size: |
180 Mb |
|
Chromosomes: |
Diploid, 3 autosomes, X and Y (2n = 8) |
|
Number of genes: |
13,000 |
|
Percentage with human homologs: |
~ 50% |
|
Average gene size: |
3 kb, 4 exons/gene |
|
Transposons: |
P elements, among others |
|
Genome sequenced in: |
2000 |
Drosophila melanogaster

Key organism for studying:
Transmission genetics
Cytogenetics
Development
Population genetics
Evolution
The fruit fly Drosophila melanogaster (loosely translated as “dusky syrup-
Special features
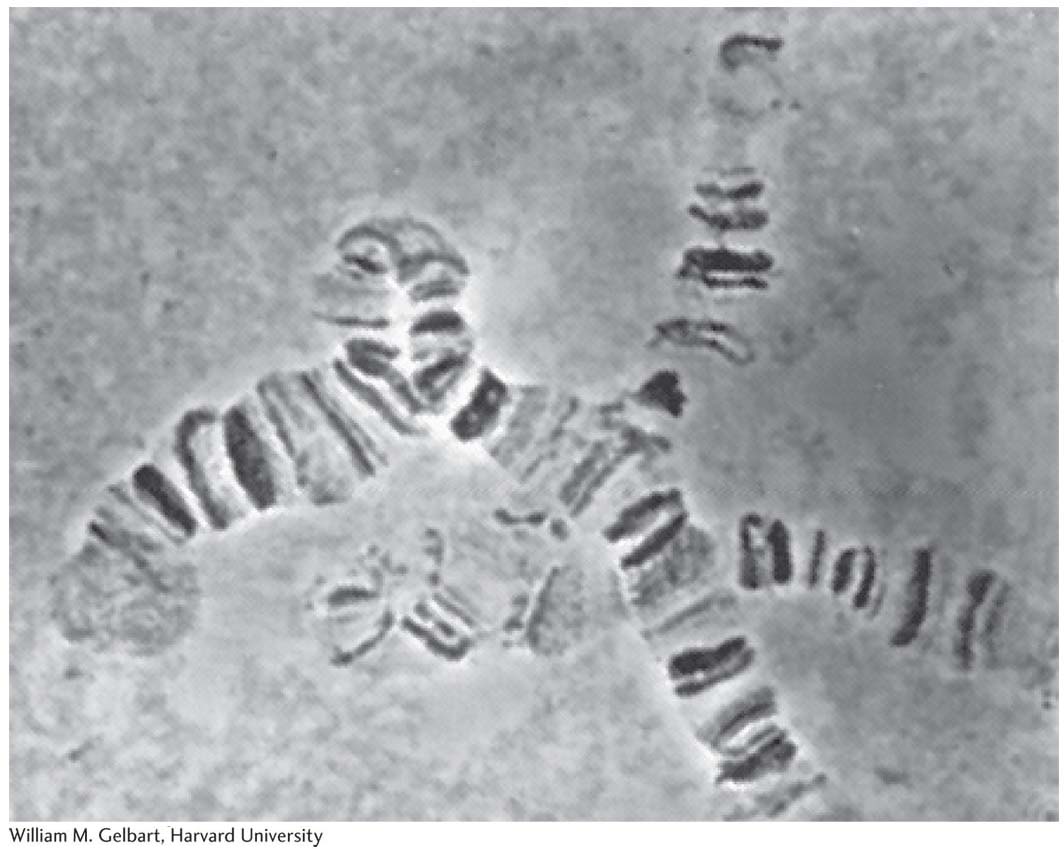
Drosophila came into vogue as an experimental organism in the early twentieth century because of features common to most model organisms. It is small (3 mm long), simple to raise (originally, in milk bottles), quick to reproduce (only 12 days from egg to adult), and easy to obtain (just leave out some rotting fruit). It proved easy to amass a large range of interesting mutant alleles that were used to lay the ground rules of transmission genetics. Early researchers also took advantage of a feature unique to the fruit fly: polytene chromosomes. In salivary glands and certain other tissues, these “giant chromosomes” are produced by multiple rounds of DNA replication without chromosomal segregation. Each polytene chromosome displays a unique banding pattern, providing geneticists with landmarks that could be used to correlate recombination-
Genetic analysis
Crosses in Drosophila can be performed quite easily. The parents may be wild or mutant stocks obtained from stock centers or as new mutant lines.
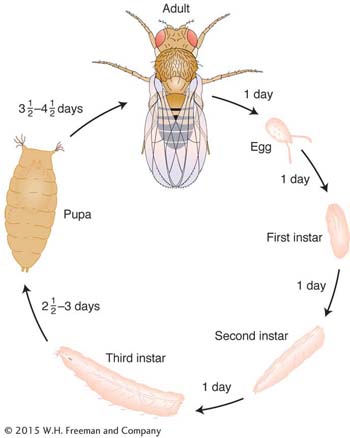
Life Cycle
Drosophila has a short diploid life cycle that lends itself well to genetic analysis. After hatching from an egg, the fly develops through several larval stages and a pupal stage before emerging as an adult, which soon becomes sexually mature. Sex is determined by X and Y sex chromosomes (XX is female, XY is male), although, in contrast with humans, the number of X’s in relation to the number of autosomes determines sex.
Total length of life cycle: 12 days from egg to adult
805
To perform a cross, males and females are placed together in a jar, and the females lay eggs in semisolid food covering the jar’s bottom. After emergence from the pupae, offspring can be anesthetized to permit counting members of phenotypic classes and to distinguish males and females (by their different abdominal stripe patterns). However, because female progeny stay virgin for only a few hours after emergence from the pupae, they must immediately be isolated if they are to be used to make controlled crosses. Crosses designed to build specific gene combinations must be carefully planned, because crossing over does not take place in Drosophila males. Hence, in the male, linked alleles will not recombine to help create new combinations.
For obtaining new recessive mutations, special breeding programs (of which the prototype is Muller’s ClB test) provide convenient screening systems. In these tests, mutagenized flies are crossed with a stock having a balancer chromosome. Recessive mutations are eventually brought to homozygosity by inbreeding for one or two generations, starting with single F1 flies.
Techniques of Genetic Modification
|
Standard mutagenesis: |
|
|
Chemical (EMS) and radiation |
Random germ- |
|
Transgenesis: |
|
|
P element mediated |
Random insertion |
|
Targeted gene knockouts: |
|
|
Induced replacement |
Null ectopic allele exits and recombines with wild- |
|
RNAi |
Mimics targeted knockout |
Genetic engineering
Transgenesis. Building transgenic flies requires the help of a Drosophila transposon called the P element. Geneticists construct a vector that carries a transgene flanked by P-element repeats. The transgene vector is then injected into a fertilized egg along with a helper plasmid containing a transposase. The transposase allows the transgene to jump randomly into the genome in germinal cells of the embryo (see Chapter 15).
Targeted knockouts. Targeted gene knockouts can be accomplished by, first, introducing a null allele transgenically into an ectopic position and, second, inducing special enzymes that cause excision of the null allele. The excised fragment (which is linear) then finds and replaces the endogenous copy by homologous crossing over. However, functional knockouts can be produced more efficiently by RNAi.
Main contributions
Much of the early development of the chromosome theory of heredity was based on the results of Drosophila studies. Geneticists working with Drosophila made key advances in developing techniques for gene mapping, in understanding the origin and nature of gene mutation, and in documenting the nature and behavior of chromosomal rearrangements.
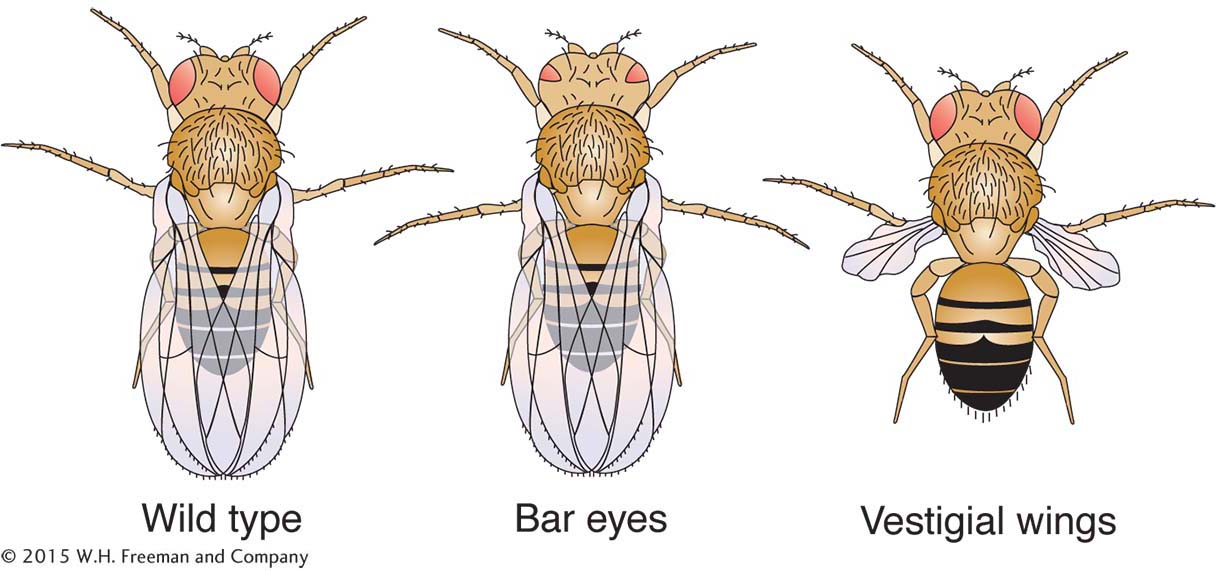
Their discoveries opened the door to other pioneering studies:
Early studies on the kinetics of mutation induction and the measurement of mutation rates were performed with the use of Drosophila. Muller’s ClB test and similar tests provided convenient screening methods for recessive mutations.
Chromosomal rearrangements that move genes adjacent to heterochromatin were used to discover and study position-
effect variegation. In the last part of the twentieth century, after the identification of certain key mutational classes such as homeotic and maternal-
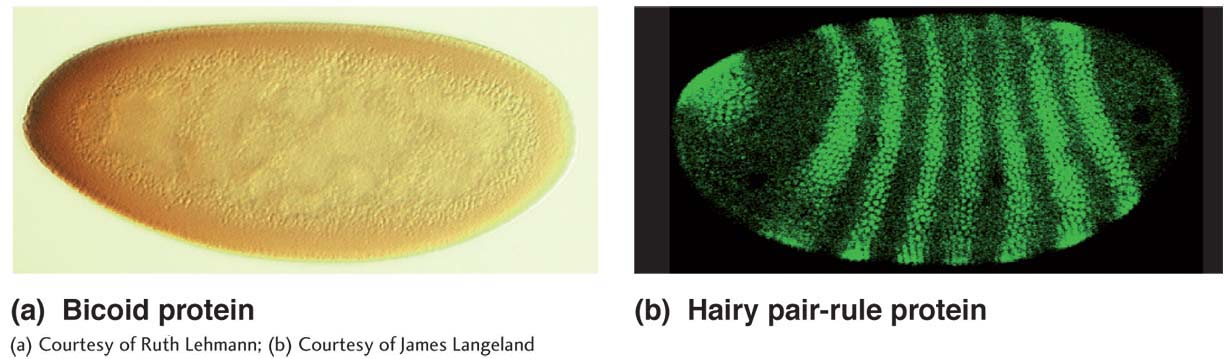
effect mutations, Drosophila assumed a central role in the genetics of development, a role that continues today (see Chapter 13). Maternal- effect mutations that affect the development of embryos, for example, have been crucial in the elucidation of the genetic determination of the Drosophila body plan; these mutations are identified by screening for abnormal developmental phenotypes in the embryos from a specific female. Techniques such as enhancer trap screens have enabled the discovery of new regulatory regions in the genome that affect development. Through these methods and others, Drosophila biologists have made important advances in understanding the determination of segmentation and of the body axes. Some of the key genes discovered, such as the homeotic genes, have widespread relevance in animals generally.
Other areas of contribution
Population genetics
Evolutionary genetics
Behavioral genetics
806