Mus musculus
| Genetic "Vital Statistics" | |
|---|---|
|
Genome size: |
2600 Mb |
|
Chromosomes: |
19 autosomes, X and Y (2n = 40) |
|
Number of genes: |
30,000 |
|
Percentage with human homologs: |
99% |
|
Average gene size: |
40 kb, 8.3 exons/gene |
|
Transposons: |
Source of 38% of genome |
|
Genome sequenced in: |
2002 |
Mus musculus

Key organism for studying:
Human disease
Mutation
Development
Coat color
Immunology

Because humans and most domesticated animals are mammals, the genetics of mammals is of great interest. However, mammals are not ideal for genetics: they are relatively large in size compared with other model organisms, thereby taking up large and expensive facilities, their life cycles are long, and their genomes are large and complex. Compared with other mammals, however, mice (Mus musculus) are relatively small, have short life cycles, and are easily obtained, making them an excellent choice for a mammal model. In addition, mice had a head start in genetics because mouse “fanciers” had already developed many different interesting lines of mice that provided a source of variants for genetic analysis. Research on the Mendelian genetics of mice began early in the twentieth century.
Special features
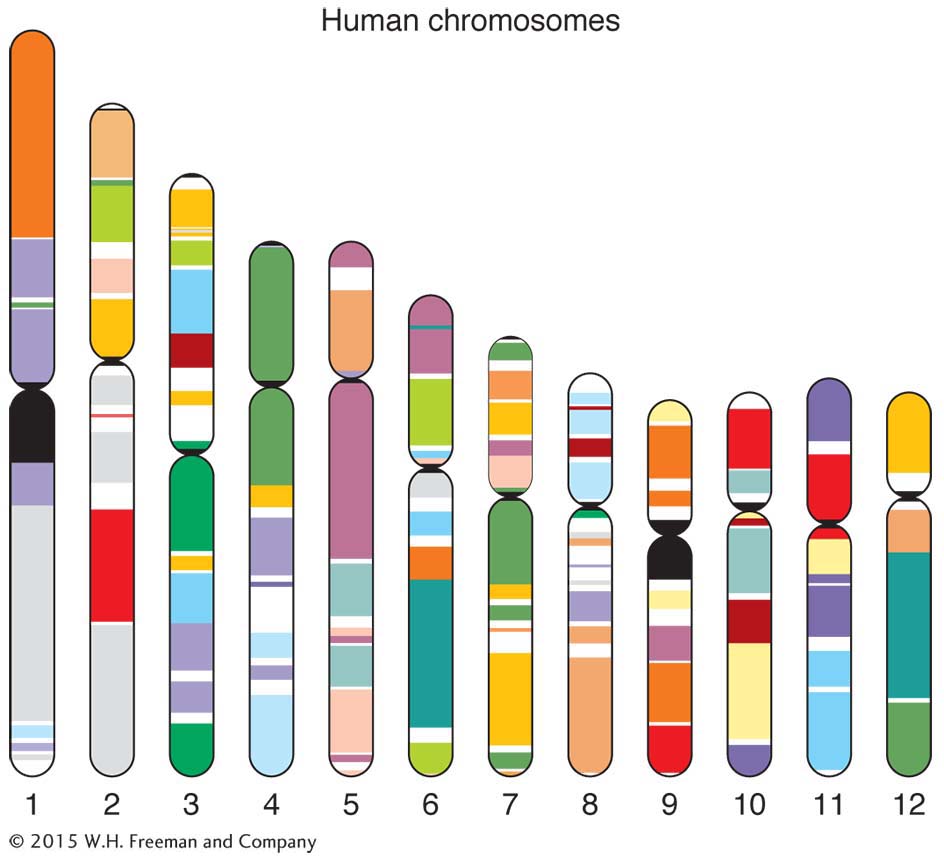
Mice are not exactly small, furry humans, but their genetic makeup is remarkably similar to ours. Among model organisms, the mouse is the one whose genome most closely resembles the human genome. The mouse genome is about 14 percent smaller than that of humans (the human genome is 3000 Mb), but it has approximately the same number of genes (current estimates are just under 30,000). A surprising 99 percent of mouse genes seem to have homologs in humans. Furthermore, a large proportion of the genome is syntenic with that of humans; that is, there are large blocks containing the same genes in the same relative positions. Such genetic similarities are the key to the mouse’s success as a model organism; these similarities allow mice to be treated as “stand-
Genetic analysis
Mutant and “wild type” (though not actually from the wild) mice are easy to come by: they can be ordered from large stock centers that provide mice suitable for crosses and various other types of experiments. Many of these lines are derived from mice bred in past centuries by mouse fanciers. Controlled crosses can be performed simply by pairing a male with a nonpregnant female. In most cases, the parental genotypes can be provided by male or female.
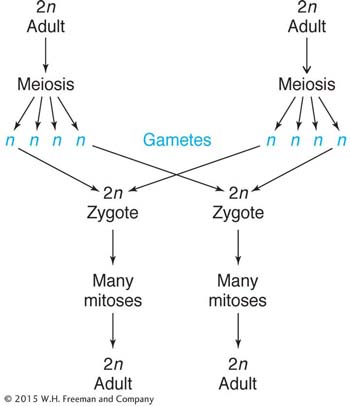
Life Cycle
Mice have a familiar diploid life cycle, with an XY sex-
Total length of life cycle: 10 weeks from birth to giving birth, in most laboratory strains
807
Most of the standard estimates of mammalian mutation rates (including those of humans) are based on measurements in mice. Indeed, mice provide the final test of agents suspected of causing mutations in humans. Mutation rates in the germ line are measured with the use of the specific-
Techniques of Genetic Modification
|
Standard mutagenesis: |
|
|
Chemicals and radiation |
Germ- |
|
Transgenesis: |
|
|
Transgene injection into zygote |
Random and homologous insertion |
|
Transgene uptake by stem cells |
Random and homologous insertion |
|
Targeted gene knockouts: |
|
|
Null transgene uptake by stem cells |
Targeted knockout stem cells selected |
Genetic engineering
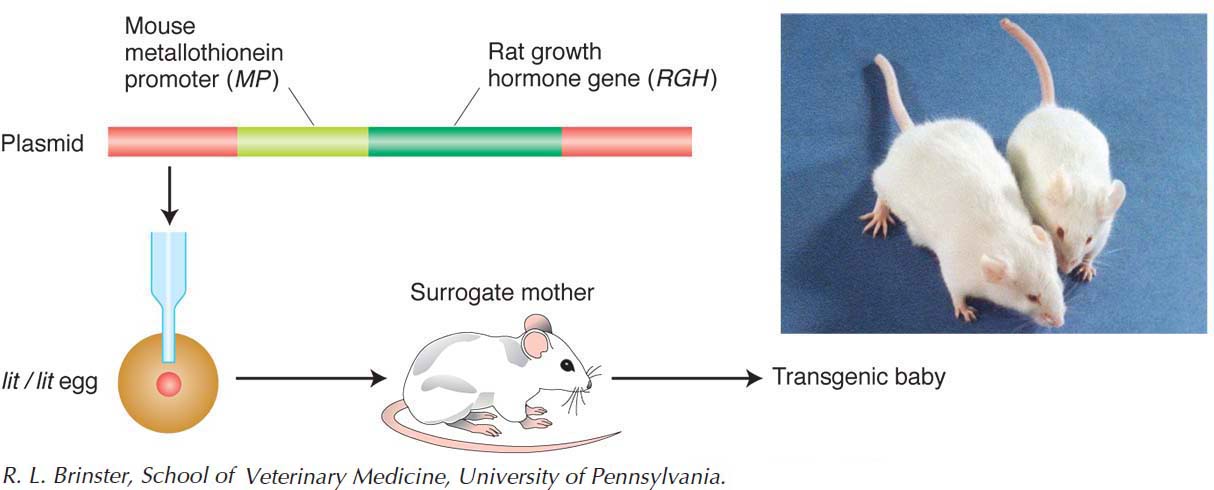
Transgenesis. The creation of transgenic mice is straightforward but requires the careful manipulation of a fertilized egg (see Chapter 10). First, mouse genomic DNA is cloned in E. coli with the use of bacterial or phage vectors. The DNA is then injected into a fertilized egg, where it integrates at ectopic (random) locations in the genome or, less commonly, at the normal locus. The activity of the transgene’s protein can be monitored by fusing the transgene with a reporter gene such as GFP before the gene is injected. With the use of a similar method, the somatic cells of mice also can be modified by transgene insertion: specific fragments of DNA are inserted into individual somatic cells and these cells are, in turn, inserted into mouse embryos.
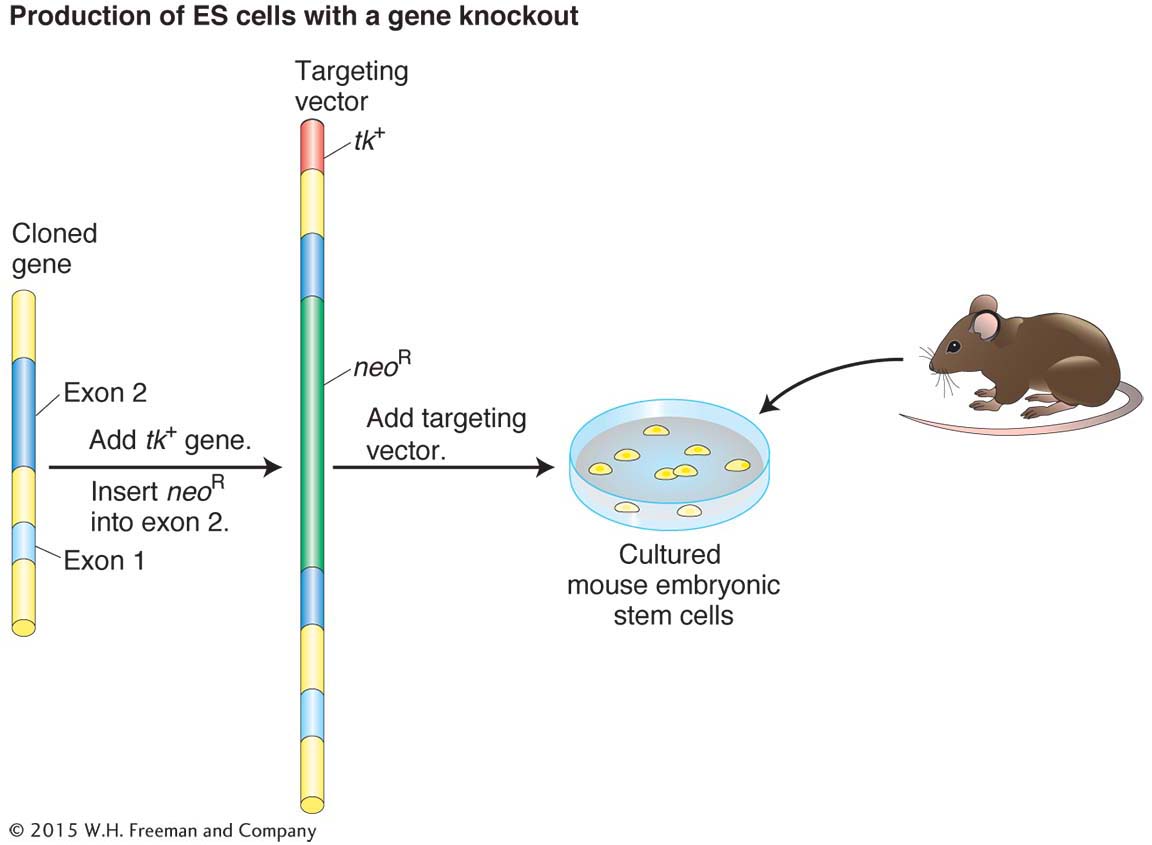
Targeted knockouts. Knockouts of specific genes for genetic dissection can be accomplished by introducing a transgene containing a defective allele and two drug-
Main contributions
Early in the mouse’s career as a model organism, geneticists used mice to elucidate the genes that control coat color and pattern, providing a model for all fur-
A large proportion of human genetic diseases have a mouse counterpart—
called a “mouse model”—useful for experimental study. Mice serve as models for the mechanisms of mammalian mutation.
Studies on the genetic mechanisms of cancer are performed on mice.
Many potential carcinogens are tested on mice.
Mice have been important models for the study of mammalian developmental genetics. For example, they provide a model system for the study of genes affecting cleft lip and cleft palate, a common human developmental disorder.
Cell lines that are fusion hybrids of mouse and human genomes played an important role in the assignment of human genes to specific human chromosomes. There is a tendency for human chromosomes to be lost from such hybrids, and so loss of specific chromosomes can be correlated with loss of specific human alleles.
Other areas of contribution
Behavioral genetics
Quantitative genetics
The genes of the immune system
808