10.6 Genetic Engineering
Thanks to recombinant-DNA technology, genes can be isolated and characterized as specific nucleotide sequences. But even this achievement is not the end of the story. We will see next that knowledge of a sequence is often the beginning of a fresh round of genetic manipulation. When characterized, a sequence can be manipulated to alter an organism’s genotype. The introduction of an altered gene into an organism has become central to basic genetic research, but it also finds wide commercial application. Two examples of the latter are (1) goats that secrete in their milk antibiotics derived from a fungus and (2) plants kept from freezing by the incorporation of arctic-fish “antifreeze” genes into their genomes. The use of recombinant-DNA techniques to alter an organism’s genotype and phenotype is termed genetic engineering, and its application to practical applications is called biotechnology.
The techniques of genetic engineering described in the first part of this chapter were originally developed in bacteria. Thus, these techniques needed to be extended to model eukaryotes, which constitute a large proportion of model research organisms. Eukaryotic genes are still typically cloned and sequenced in bacterial hosts, but eventually they are introduced into a eukaryote, either the original donor species or a completely different one. The gene transferred is called a transgene, and the engineered product is called a transgenic organism.
The transgene can be introduced into a eukaryotic cell by a variety of techniques, including transformation, injection, bacterial or viral infection, and bombardment with DNA-coated tungsten or gold particles using a gene gun (Figure 10-22). When the transgene enters a cell, it travels to the nucleus, where it must become a stable part of the genome by either inserting into a chromosome or (in a few species only) replicating as part of a plasmid. If insertion occurs, the transgene can either replace the resident gene by homologous recombination or insert ectopically—that is, at other locations in the genome. Transgenes from other species typically insert ectopically.
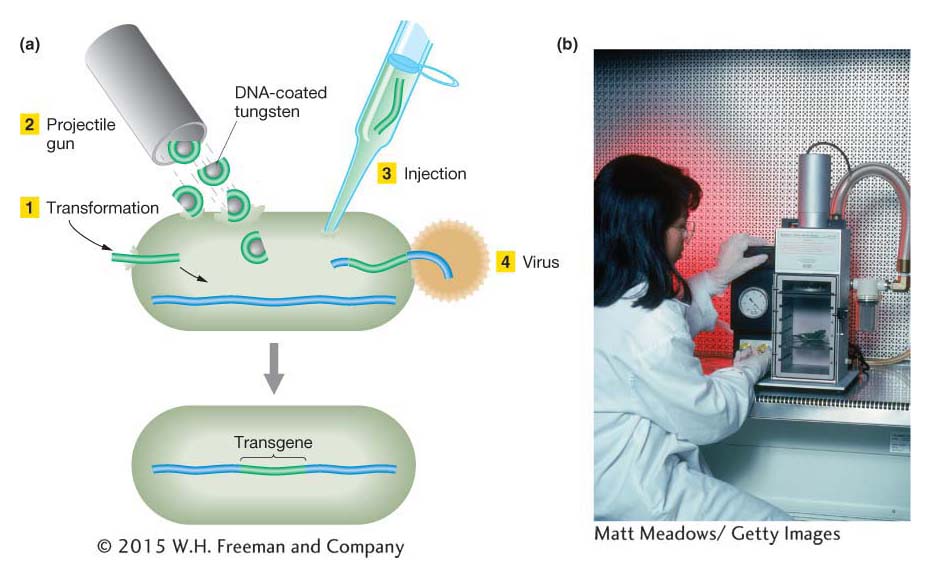
Figure 10-22: Methods of introducing a transgene
Figure 10-22: (a) Four different ways to introduce foreign DNA into a cell. (b) A gene gun.
[(b) Matt Meadows/Getty Images.]
KEY CONCEPT
Transgenesis can introduce new or modified genetic material into eukaryotic cells.We now turn to some examples in fungi, plants, and animals.
Genetic engineering in Saccharomyces cerevisiae
It is fair to say that S. cerevisiae is the most sophisticated easily manipulated eukaryotic genetic model. Most of the techniques typically used for eukaryotic genetic engineering were developed in yeast; so let’s consider the general routes for transgenesis in yeast.
The simplest yeast vectors are yeast integrative plasmids (YIps), derivatives of bacterial plasmids into which the yeast DNA of interest has been inserted. When transformed into yeast cells, these plasmids insert into yeast chromosomes, generally by homologous recombination with the resident gene, by either a single or a double crossover (Figure 10-23). As a result, either the entire plasmid is inserted or the targeted allele is replaced by the allele on the plasmid. The latter is an example of gene replacement—in this case, the substitution of an engineered gene for the gene originally in the yeast cell. Gene replacement can be used to delete a gene or substitute a mutant allele for its wild-type counterpart or, conversely, to substitute a wild-type allele for a mutant. Such substitutions can be detected by plating cells on a medium that selects for a marker allele on the plasmid.
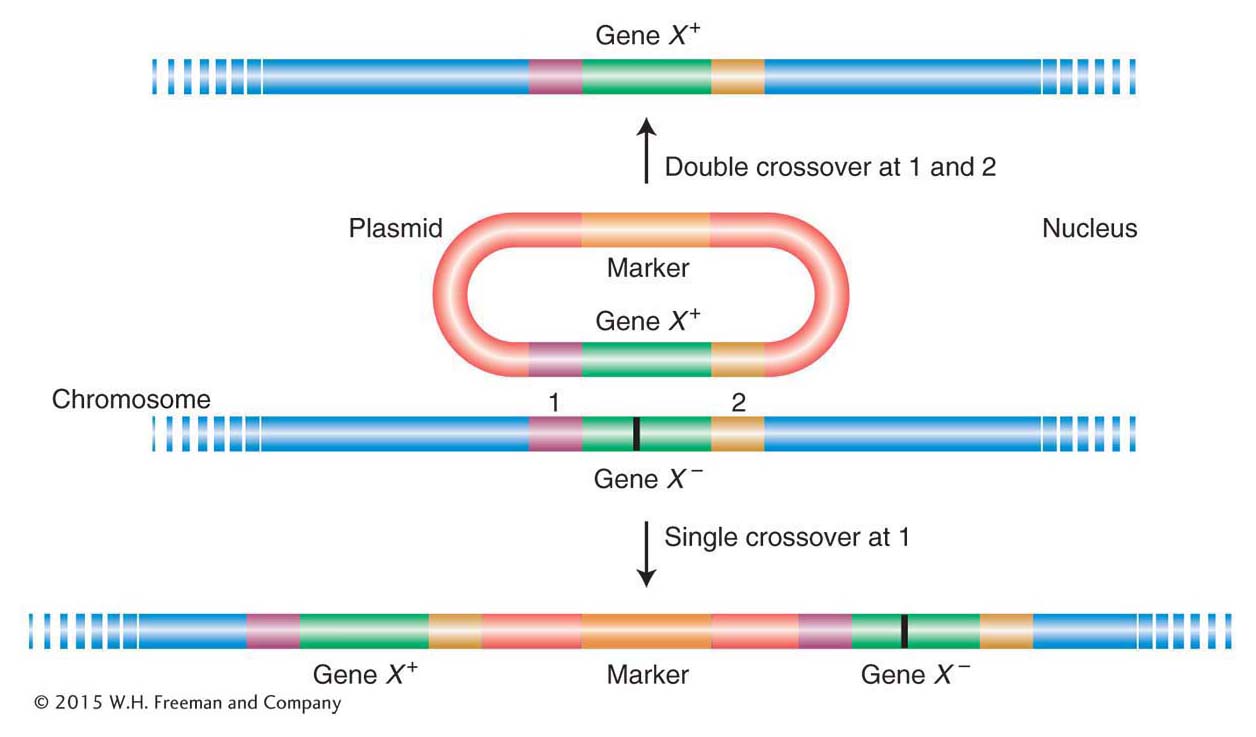
Figure 10-23: Two results of transformation by simple yeast vectors
Figure 10-23: A plasmid bearing an active allele (gene X+) inserts into a recipient yeast strain bearing a defective gene X−) by homologous recombination. The result can be replacement of the defective gene X− by X+ (top) or its retention along with the new allele (bottom). The mutant site of gene X− is represented as a vertical black bar. Single crossovers at position 2 also are possible but are not shown.
The bacterial origin of replication is different from eukaryotic origins, and so bacterial plasmids do not replicate in yeast. Therefore, the only way in which such vectors can generate a stable modified genotype is if they are integrated into the yeast chromosome.
Genetic engineering in plants
Recombinant DNA technology has introduced a new dimension to the effort to develop improved crop varieties. No longer is genetic diversity achieved solely by selecting variants within a given species. DNA can now be introduced from other species of plants, animals, or even bacteria, producing genetically modified organisms (GMOs). The genome modifications made possible by this technology are almost limitless. In response to new possibilities, a sector of the public has expressed concern that the introduction of GMOs into the food supply may produce unexpected health problems. The concern about GMOs is one facet of an ongoing public debate about complex public health, safety, ethical, and educational issues raised by the new genetic technologies.
A vector routinely used to produce transgenic plants is derived from the Ti plasmid, a natural plasmid from a soil bacterium called Agrobacterium tumefaciens. This bacterium causes what is known as crown gall disease, in which the infected plant produces uncontrolled growths called tumors or galls. The key to tumor production is a large (200-kb) circular DNA plasmid—the Ti (tumor-inducing) plasmid. When the bacterium infects a plant cell, a part of the Ti plasmid is transferred and inserted, apparently more or less at random, into the genome of the host plant (Figure 10-24). The region of the Ti plasmid that inserts into the host plant is called T-DNA, for transfer DNA. The genes whose products catalyze this T-DNA transfer reside in a region of the Ti plasmid separate from the T-DNA region itself.
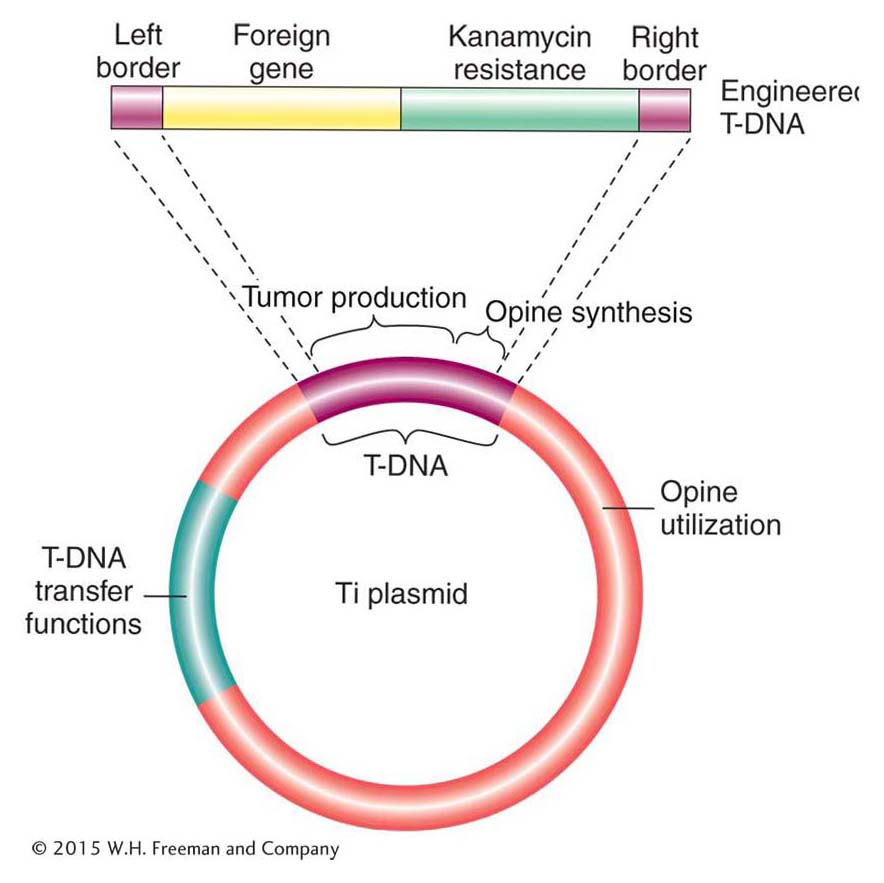
Figure 10-24: The Ti plasmid vector
Figure 10-24: Simplified representation of the major regions of the Ti plasmid of A. tumefaciens containing an engineered T-DNA.
The natural behavior of the Ti plasmid makes it well suited to the role of a vector for plant genetic engineering. In particular, any DNA that is inserted between the T-DNA border (24-bp ends) sequences can be mobilized by other functions provided by the Ti plasmid and inserted into plant chromosomes. Thus, scientists were able to eliminate all of the T-DNA sequence between the borders (including the tumor-causing genes) and replace it with the gene(s) of interest and a selectable marker (for example, kanamycin resistance). One method of introducing the T-DNA into the plant genome is shown in Figure 10-25. Bacteria containing this and similarly engineered T-DNA are used to infect cut segments of plant tissue, such as punched-out leaf disks. If the leaf disks are placed on a medium containing kanamycin, only the plant cells that have acquired the kanR gene engineered into the T-DNA will undergo cell division. The transformed cells grow into a clump, or callus, that can be induced to form shoots and roots. These calli are transferred to soil, where they develop into transgenic plants. Typically, only a single copy of the T-DNA region inserts into a given plant genome, where it segregates at meiosis like a regular Mendelian allele (Figure 10-26). The presence of the insert can be verified by screening the transgenic tissue for transgenic genetic markers or by screening purified DNA with a T-DNA probe in a Southern hybridization.
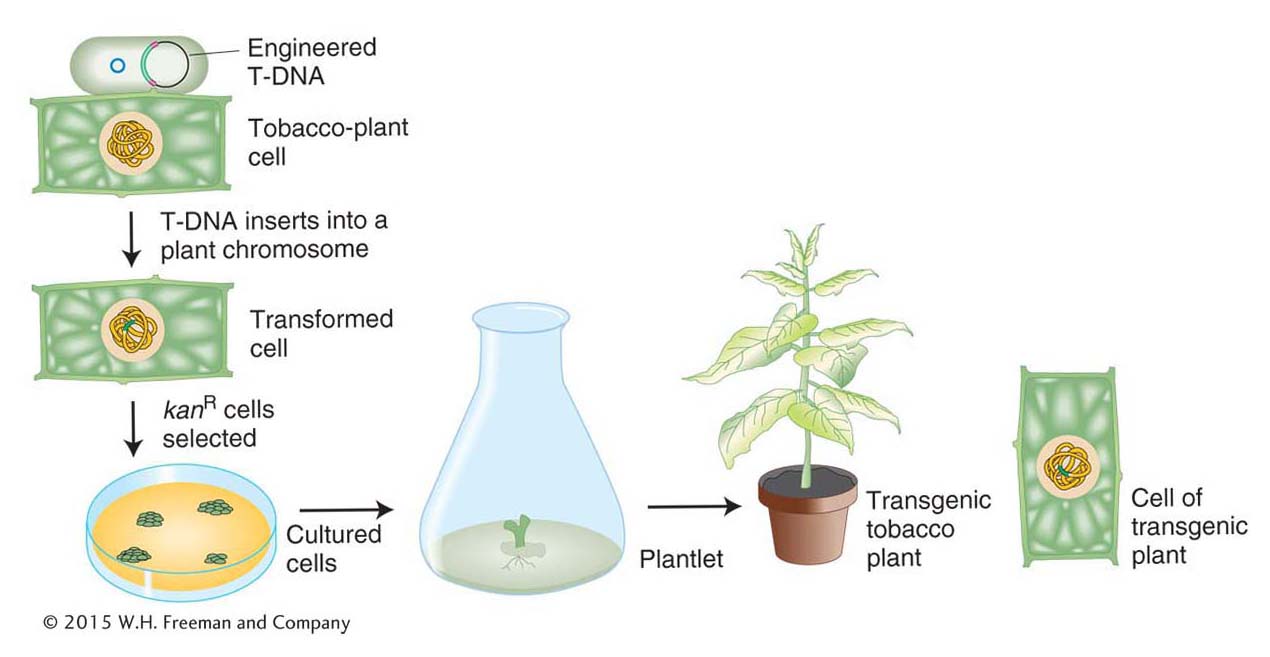
Figure 10-25: The generation of a transgenic plant
Figure 10-25: The insertion of T-DNA into plant chromosomes. Incubation of leaf disks with the bacterium A. tumefaciens containing an engineered T-DNA leads to leaf cells with the T-DNA in their genome, which are able to grow on agar plates and can be coaxed to differentiate into transgenic tobacco plants.
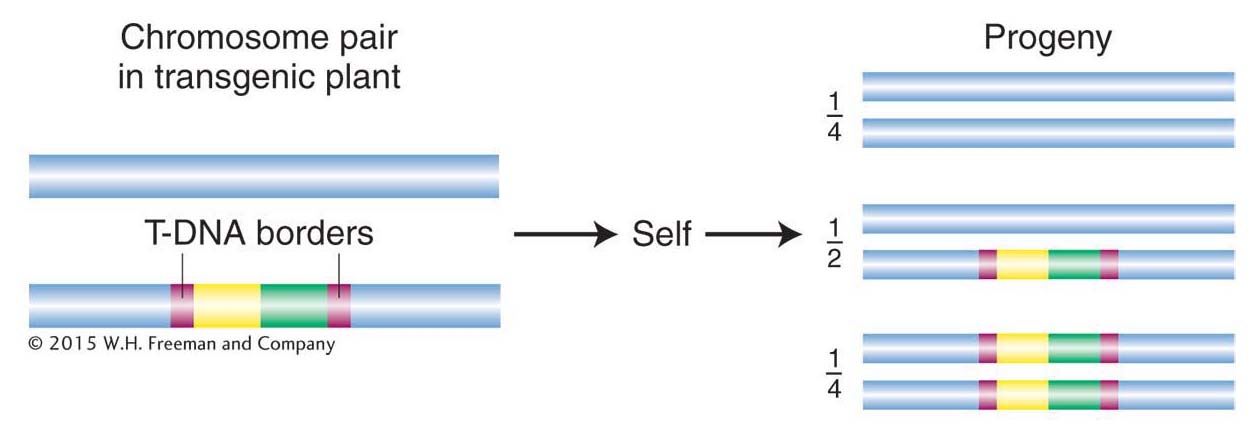
Figure 10-26: Pattern of transmission of T-DNA
Figure 10-26: The T-DNA region and any DNA inserted into a plant chromosome in a transgenic plant are transmitted in a Mendelian pattern of inheritance.
Transgenic plants carrying any one of a variety of foreign genes are in current use, including crop plants carrying genes that confer resistance to certain bacterial or fungal pests, and many more are in development. Not only are the qualities of plants themselves being manipulated, but, like microorganisms, plants are also being used as convenient “factories” to produce proteins encoded by foreign genes.
Genetic engineering in animals
Transgenic technologies are now being employed with many animal-model systems. We will focus on the two animal models heavily used for basic genetic research: the nematode Caenorhabditis elegans and the mouse Mus musculus. A commonly used method to transform a third model organism, the fruit fly Drosophila melanogaster, is described in Chapter 15. Versions of many of the techniques considered so far can also be applied in these animal systems.
Transgenesis in C. elegans The method used to introduce trangenes into C. elegans is simple: transgenic DNAs are injected directly into the organism, typically as plasmids, fosmids, or other DNAs cloned in bacteria. The injection strategy is determined by the worm’s reproductive biology. The gonads of the worm are syncytial, meaning that there are many nuclei within the same gonadal cell. One syncytial cell is a large proportion of one arm of the gonad, and the other syncytial cell is the bulk of the other arm (Figure 10-27a). These nuclei do not form individual cells until meiosis, when they begin their transformation into individual eggs or sperm. A solution of DNA is injected into the syncytial region of one of the arms, thereby exposing more than 100 nuclei to the transforming DNA. By chance, a few of these nuclei will incorporate the DNA (remember, the nuclear membrane breaks down in the course of division, and so the cytoplasm into which the DNA is injected becomes continuous with the nucleoplasm). Typically, the transgenic DNA forms multicopy extrachromosomal arrays (Figure 10-27b) that exist as independent units outside the chromosomes. More rarely, the transgenes will become integrated into an ectopic position in a chromosome, still as a multicopy array. Unfortunately, sequences may become scrambled within the arrays, complicating the work of the researcher.
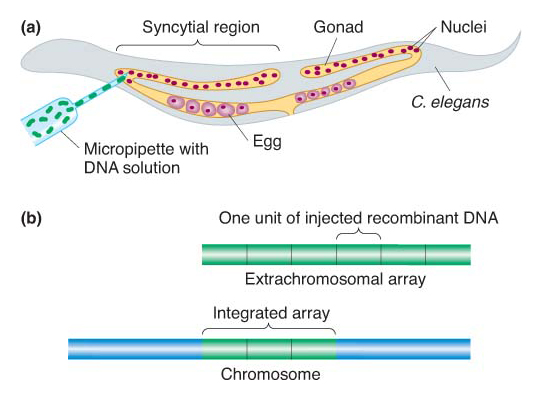
Figure 10-27: Creation of Caenorhabditis elegans transgenes
Figure 10-27: C. elegans transgenes are created by injecting transgenic DNA directly into a gonad. (a) Method of injection. (b) The two main types of transgenic results: extrachromosomal arrays and arrays integrated in ectopic chromosomal locations.
Transgenesis in M. musculus Mice are the most important models for mammalian genetics. Most exciting, much of the technology developed in mice is potentially applicable to humans. There are two strategies for transgenesis in mice, each having its advantages and disadvantages:
Ectopic insertions. Transgenes are inserted randomly in the genome, usually as multicopy arrays.
Gene targeting. The transgene sequence is inserted into a location occupied by a homologous sequence in the genome. That is, the transgene replaces its normal homologous counterpart.
Ectopic insertions To insert transgenes in random locations, the procedure is simply to inject a solution of bacterially cloned DNA into the nucleus of a fertilized egg (Figure 10-28a). Several injected eggs are inserted into the female oviduct, where some will develop into baby mice. At some later stage, the transgene becomes integrated into the chromosomes of random nuclei. On occasion, the transgenic cells form part of the germ line, and, in these cases, an injected embryo will develop into an adult mouse whose germ cells contain the transgene inserted at some random position in one of the chromosomes (Figure 10-28b). Some of the progeny of these adults will inherit the transgene in all cells. There will be an array of multiple gene copies at each point of insertion, but the location, size, and structure of the arrays will be different for each integration event. The technique does give rise to some problems: (1) the expression pattern of the randomly inserted genes may be abnormal (called a position effect) because the local chromosome environment lacks the gene’s normal regulatory sequences (see Chapter 12 for more on position effect), and (2) DNA rearrangements can occur inside the multicopy arrays (in essence, mutating the sequences). Nonetheless, this technique is much more efficient and less laborious than gene targeting.
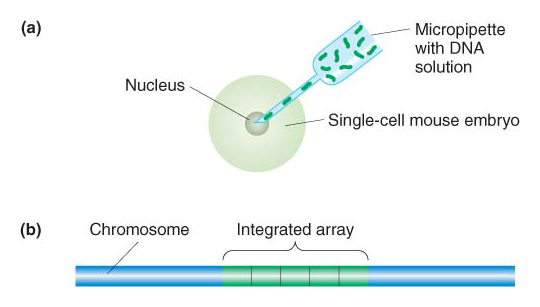
Figure 10-28: Creation of Mus musculus transgenes
Figure 10-28: M. musculus transgenes are created by injection of cloned DNA into fertilized eggs and subsequent insertion in ectopic chromosomal locations. (a) Method of injection. (b) A typical ectopic integrant, with multiple copies of the recombinant transgene inserted in an array.
Gene targeting Gene targeting enables researchers to eliminate a gene or modify the function it encodes. In one application, called gene replacement, a mutant allele can be repaired by substituting a wild-type allele for the mutant one in its normal chromosomal location. Gene replacement avoids both the position effect and the DNA rearrangements associated with ectopic insertion because a single copy of the gene is inserted in its normal chromosomal environment. Conversely, a gene may be inactivated by substituting an inactive gene for the normal gene. Such a targeted inactivation is called a gene knockout.
Gene targeting in the mouse is carried out in cultured embryonic stem cells (ES cells). In general, a stem cell is an undifferentiated cell in a given tissue or organ that divides asymmetrically to produce a progeny stem cell and a cell that will differentiate into a terminal cell type. ES cells are special stem cells that can differentiate to form any cell type in the body—including, most importantly, the germ line.
To illustrate the process of gene targeting, we look at how it achieves one of its typical outcomes—namely, the substitution of an inactive gene for the normal gene, or gene knockout. The process requires three stages:
An inactive gene is targeted to replace the functioning gene in a culture of ES cells, producing ES cells containing a gene knockout (Figure 10-29).
ES cells containing the inactive gene are transferred to mice embryos (Figure 10-30).
Transgenic mice are identified and bred to produce mice of known genotype.
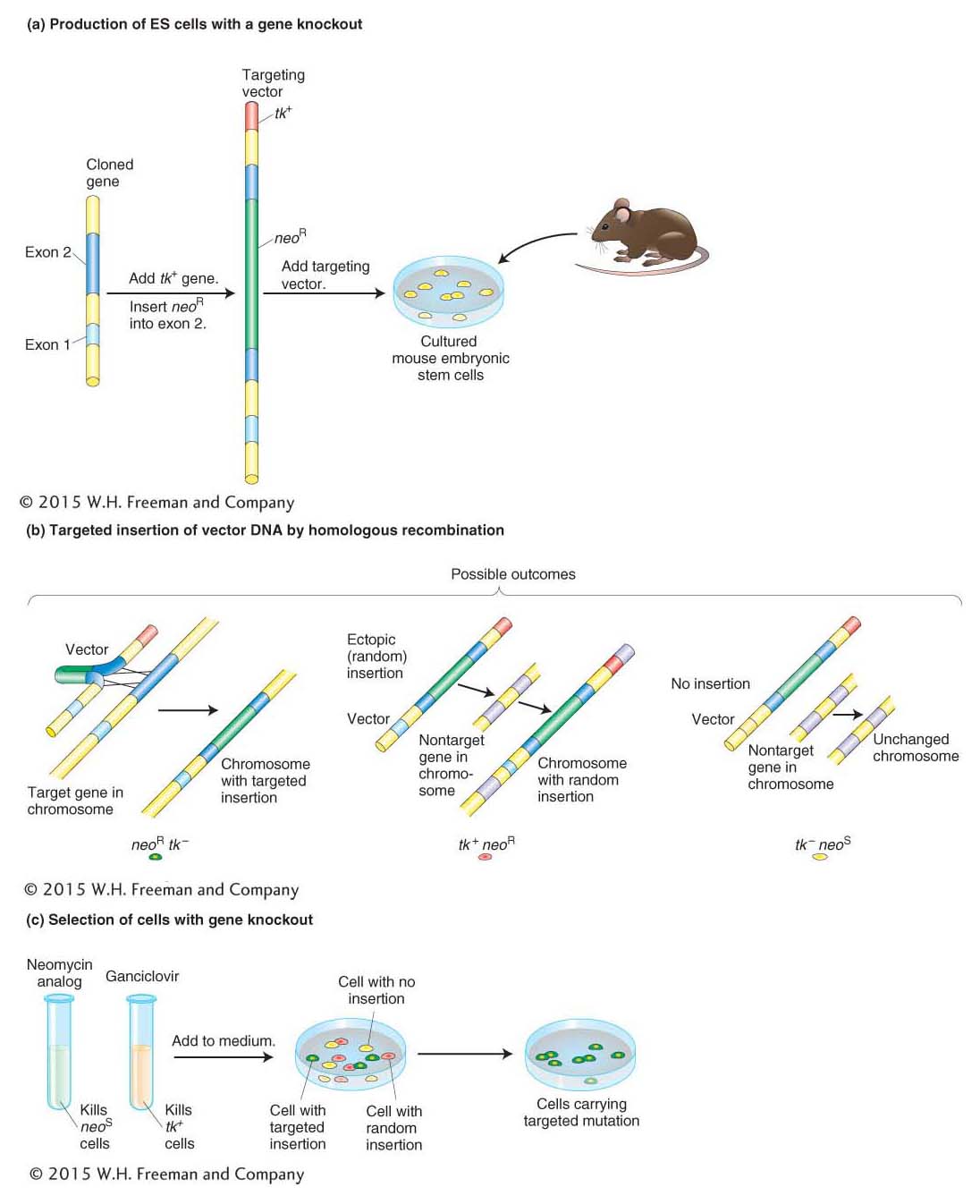
Figure 10-29: Producing cells containing a targeted gene knockout
Figure 10-29: Producing cells that contain a mutation in one specific gene, known as a targeted mutation or a gene knockout. (a) Copies of a cloned gene are altered in vitro to produce the targeting vector. The gene shown here was inactivated by the insertion of the neomycin-resistance gene (neoR) into a protein-coding region (exon 2) of the gene and had been inserted into a vector. The neoR gene will serve later as a marker to indicate that the vector DNA took up residence in a chromosome. The vector was also engineered to carry a second marker at one end: the herpes tk gene. These markers are standard, but others could be used instead. When a vector, with its dual markers, is complete, it is introduced into cells isolated from a mouse embryo. (b) When homologous recombination occurs (left), the homologous regions on the vector, together with any DNA in between but excluding the marker at the tip, take the place of the original gene. This event is important because the vector sequences serve as a useful tag for detecting the presence of this mutant gene. In many cells, though, the full vector (complete with the extra marker at the tip) inserts ectopically (middle) or does not become integrated at all (right). (c) To isolate cells carrying a targeted mutation, all the cells are put into a medium containing selected drugs—here, a neomycin analog (G418) and ganciclovir. G418 is lethal to cells unless they carry a functional neoR gene, and so it eliminates cells in which no integration of vector DNA has taken place (yellow). Meanwhile, ganciclovir kills any cells that harbor the tk gene, thereby eliminating cells bearing a randomly integrated vector (red). Consequently, virtually the only cells that survive and proliferate are those harboring the targeted insertion (green).
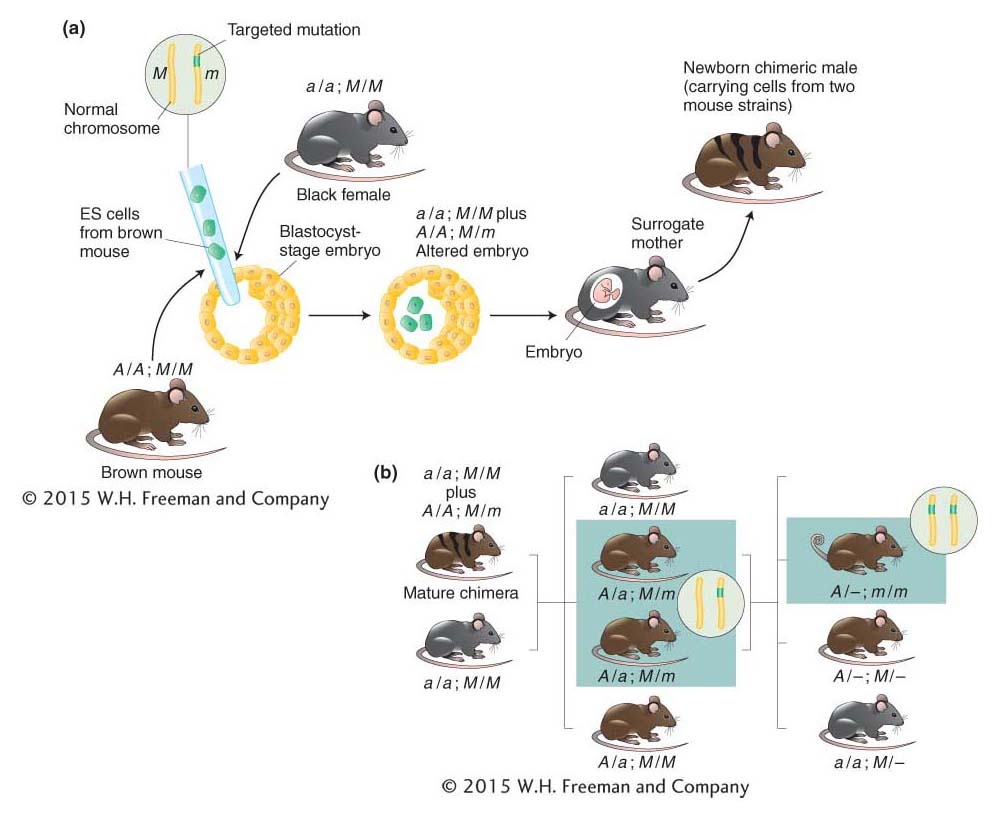
Figure 10-30: Producing a mouse containing the targeted gene knockout
Figure 10-30: A knockout mouse is produced by inserting ES cells carrying the targeted mutation. (a) Embryonic stem (ES) cells are isolated from an agouti (brown) mouse strain (A/A) and altered to carry a targeted mutation (m) in one chromosome. The ES cells are then inserted into young embryos, one of which is shown. Coat color of the future newborns is a guide to whether the ES cells have survived in the embryo. Hence, ES cells are typically put into embryos that, in the absence of the ES cells, would acquire a totally black coat. Such embryos are obtained from a black strain that lacks the dominant agouti allele (a/a). The embryos containing the ES cells grow to term in surrogate mothers. Agouti shading intermixed with black indicates those newborns in which the ES cells have survived and proliferated. (Such mice are called chimeras because they contain cells derived from two different strains of mice.) Solid black coloring, in contrast, indicates that the ES cells have perished, and these mice are excluded. A represents agouti; a, black; m is the targeted mutation; and M is its wild-type allele. (b) Chimeric males are mated with black (nonagouti) females. Progeny are screened for evidence of the targeted mutation (green in inset) in the gene of interest. Direct examination of the genes in the agouti mice reveals which of those animals (boxed) inherited the targeted mutation. Males and females carrying the mutation are mated with one another to produce mice whose cells carry the chosen mutation in both copies of the target gene (inset) and thus lack a functional gene. Such animals (boxed) are identified definitively by direct analyses of their DNA. The knockout in this case results in a curly-tail phenotype.
Stage 1: The inactive version of the gene is prepared by inserting a DNA segment that disrupts copies of the cloned gene. Then DNA constructs containing the defective gene are injected into the nuclei of cultured ES cells. The defective gene inserts far more frequently into nonhomologous (ectopic) sites than into homologous sites (Figure 10-29b), and so the next step is to select the rare cells in which the defective gene has replaced the functioning gene as desired. How is it possible to select ES cells that contain a rare gene replacement? The genetic engineer can include drug-resistance alleles in the DNA construct arranged in such a way that replacements can be distinguished from ectopic insertions. An example is shown in Figure 10-29c.
Stage 2: The ES cells that contain one copy of the disrupted gene of interest (that is, gene knockout) are injected into a blastocyst-stage embryo, which is then implanted in a surrogate mother (Figure 10-30a). Some of the ES cells may become incorporated into the host embryo, and if that happens, the mouse that develops will be chimeric—that is, it will contain cells from two different mouse strains. When the chimeric mouse reaches adulthood, it is mated with a normal mouse. If the chimeric mouse had taken up ES cells (with the knockout gene) into germ-line cells, then some of the resulting offspring will inherit the gene knockout in all their cells. Sibling mice that are identified as being heterozygous for the knockout version of the gene of interest are then mated in order to produce mice that are homozygous for the knockout allele. (If the gene is essential, homozygotes will be lethal and none will be obtained from this cross.) (Figure 10-30b).
KEY CONCEPT
Germ-line transgenic techniques have been developed for all well-studied eukaryotic species. These techniques depend on an understanding of the reproductive biology of the recipient species.








