11.2 Discovery of the lac System: Negative Control
To study gene regulation, ideally we need three ingredients: a biochemical assay that lets us measure the amount of mRNA or expressed protein or both, reliable conditions in which the levels of expression differ in a wild-type genotype, and genetic mutations that perturb the levels of expression. In other words, we need a way of describing wild-type gene regulation and we need mutations that can disrupt the wild-type regulatory process. With these elements in hand, we can analyze the expression in mutant genotypes, treating the mutations singly and in combination, to unravel any kind of gene-regulation event. The classical application of this approach was used by Jacob and Monod, who performed the definitive studies of bacterial gene regulation.
Jacob and Monod used the lactose metabolism system of E. coli (see Figure 11-4) to genetically dissect the process of enzyme induction—that is, the appearance of a specific enzyme only in the presence of its substrates. This phenomenon had been observed in bacteria for many years, but how could a cell possibly “know” precisely which enzymes to synthesize? How could a particular substrate induce the appearance of a specific enzyme?
In the lac system, the presence of lactose causes cells to produce more than 1000 times as much of the enzyme β-galactosidase as they produced when grown in the absence of lactose. What role did lactose play in the induction phenomenon? One idea was that lactose was simply activating a precursor form of β-galactosidase that had accumulated in the cell. However, when Monod and co-workers followed the fate of radioactively labeled amino acids added to growing cells either before or after the addition of an inducer, they found that induction resulted in the synthesis of new enzyme molecules, as indicated by the presence of the radioactive amino acids in the enzymes. These new molecules could be detected as early as 3 minutes after the addition of an inducer. Additionally, withdrawal of lactose brought about an abrupt halt in the synthesis of the new enzyme. Therefore, it became clear that the cell has a rapid and effective mechanism for turning gene expression on and off in response to environmental signals.
Genes controlled together
When Jacob and Monod induced β-galactosidase, they found that they also induced the enzyme permease, which is required to transport lactose into the cell. The analysis of mutants indicated that each enzyme was encoded by a different gene. The enzyme transacetylase (with a dispensable and as yet unknown function) also was induced together with β-galactosidase and permease and was later shown to be encoded by a separate gene. Therefore, Jacob and Monod could identify three coordinately controlled genes. Recombination mapping showed that the Z, Y, and A genes were very closely linked on the chromosome.
Genetic evidence for the operator and repressor
Now we come to the heart of Jacob and Monod’s work: How did they deduce the mechanisms of gene regulation in the lac system? Their strategy was a classic genetic approach: to examine the physiological consequences of mutations. Thus, they induced mutations in the structural genes and regulatory elements of the lac operon. As we will see, the properties of mutations in these different components of the lac operon are quite different, providing important clues for Jacob and Monod.
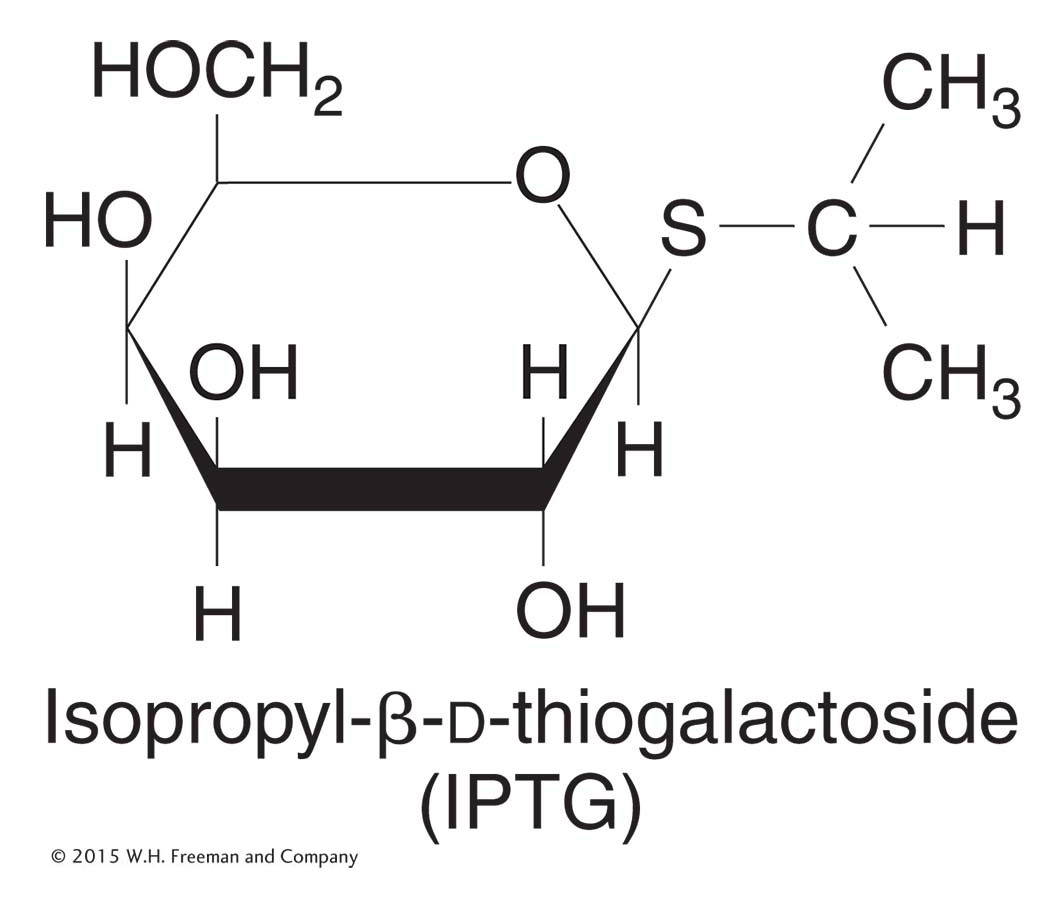
Figure 11-7: Structure of IPTG
Figure 11-7: IPTG is an inducer of the lac operon.
Natural inducers, such as allolactose, are not optimal for these experiments because they are broken down by β-galactosidase. The inducer concentration decreases during the experiment, and so the measurements of enzyme induction become quite complicated. Instead, for such experiments, Jacob and Monod used synthetic inducers, such as isopropyl-β-d-thiogalactoside (IPTG; Figure 11-7). IPTG is not hydrolyzed by β-galactosidase, but it still induces β-galactosidase enzyme expression.
Jacob and Monod found that several different classes of mutations can alter the expression of the structural genes of the lac operon. They were interested in assessing the interactions between the new alleles, such as which alleles exhibited dominance. But to perform such tests, one needs diploids, and bacteria are haploid. However, Jacob and Monod were able to produce bacteria that are partially diploid by inserting F′ factors (see Chapter 5) carrying the lac region of the genome. They could then create strains that were heterozygous for selected lac mutations. These partial diploids allowed Jacob and Monod to distinguish mutations in the regulatory DNA site (the lac operator) from mutations in the regulatory protein (the Lac repressor encoded by the I gene).
We begin by examining mutations that inactivate the structural genes for β-galactosidase and permease (designated Z− and Y−, respectively). The first thing that we learn is that Z− and Y− are recessive to their respective wild-type alleles (Z+ and Y+). For example, strain 2 in Table 11-1 can be induced to synthesize β-galactosidase (like the wild-type haploid strain 1 in this table) even though it is heterozygous for mutant and wild-type Z alleles. This demonstrates that the Z+ allele is dominant over its Z− counterpart.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Note: Bacteria were grown in glycerol (no glucose present) with and without the inducer IPTG. The presence or absence of enzyme is indicated by + or −, respectively. All strains are l+. |
Table 11-1: Synthesis of β-Galactosidase and Permease in Haploid and Heterozygous Diploid Operator Mutants
Jacob and Monod first identified two classes of regulatory mutations, called OC and I−. These were called constitutive mutations because they caused the lac operon structural genes to be expressed regardless of whether inducer was present. Jacob and Monod identified the existence of the operator on the basis of their analysis of the OC mutations. These mutations make the operator incapable of binding to repressor; they damage the switch such that the operon is always “on” (Table 11-1, strain 3). Importantly, the constitutive effects of OC mutations were restricted solely to those lac structural genes on the same chromosome as the OC mutation. For this reason, the operator mutant was said to be cis-acting, as demonstrated by the phenotype of strain 4 in Table 11-1. Here, because the wild-type permease (Y+) gene is cis to the wild-type operator, permease is expressed only when lactose or an analog is present. In contrast, the wild-type β-galactosidase (Z+) gene is cis to the OC mutant operator; hence, β-galactosidase is expressed constitutively. This unusual property of cis action suggested that the operator is a segment of DNA that influences only the expression of the structural genes linked to it (Figure 11-8). The operator thus acts simply as a protein-binding site and makes no gene product.
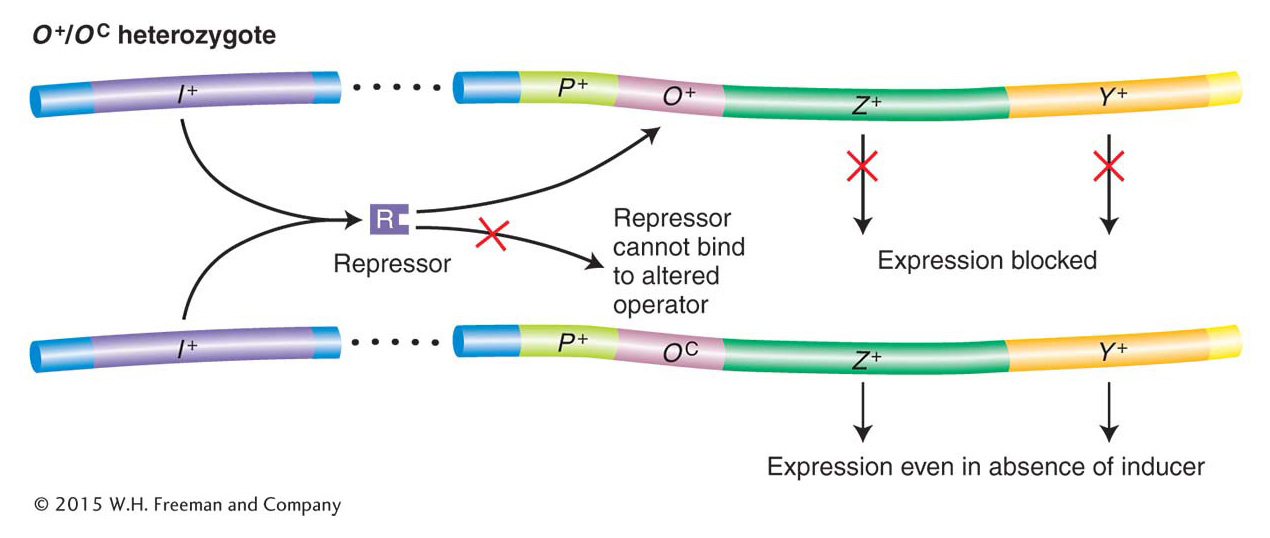
Figure 11-8: Operators are cis-acting
Figure 11-8: O+/OC heterozygotes demonstrate that operators are cis-acting. Because a repressor cannot bind to OC operators, the lac structural genes linked to an OC operator are expressed even in the absence of an inducer. However, the lac genes adjacent to an O+ operator are still subject to repression. ANIMATED ART: OC lac operator mutations
Jacob and Monod did comparable genetic tests with the I− mutations (Table 11-2). A comparison of the inducible wild-type I+ (strain 1) with I− strains shows that I− mutations are constitutive (strain 2). That is, they cause the structural genes to be expressed at all times. Strain 3 demonstrates that the inducible phenotype of I+ is dominant over the constitutive phenotype of I−. This finding showed Jacob and Monod that the amount of wild-type protein encoded by one copy of the gene is sufficient to regulate both copies of the operator in a diploid cell. Most significantly, strain 4 showed them that the I+ gene product is trans-acting, meaning that the gene product can regulate all structural lac operon genes, whether residing on the same DNA molecule or on different ones (in cis or in trans, respectively). Unlike the operator, the I gene behaves like a standard protein-coding gene. The protein product of the I gene is able to diffuse throughout a cell and act on both operators in the partial diploid (Figure 11-9).
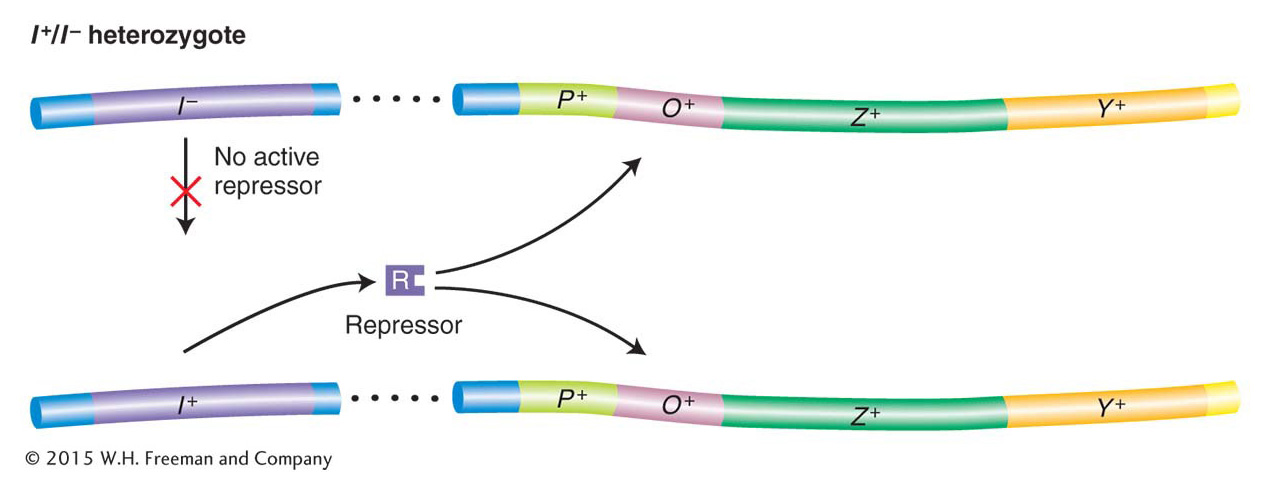
Figure 11-9: Repressors are trans-acting
Figure 11-9: The recessive nature of I− mutations demonstrates that the repressor is trans-acting. Although no active repressor is synthesized from the I− gene, the wild-type gene provides a functional repressor that binds to both operators in a diploid cell and blocks lac operon expression (in the absence of an inducer). ANIMATED ART: I− Lac repressor mutations
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Note: Bacteria were grown in glycerol (no glucose present) and induced with IPTG. The presence of the maximal level of the enzyme is indicated by a plus sign; the absence or very low level of an enzyme is indicated by a minus sign. (All strains are O+.) |
Table 11-2: Synthesis of β-Galactosidase and Permease in Haploid and Heterozygous Diploid Strains Carrying l+ and l−
KEY CONCEPT
Operator mutations reveal that such a site is cis-acting; that is, it regulates the expression of an adjacent transcription unit on the same DNA molecule. In contrast, mutations in the gene encoding a repressor protein reveal that this protein is trans-acting; that is, it can act on any copy of the target DNA.
Genetic evidence for allostery
Finally, Jacob and Monod were able to demonstrate allostery through the analysis of another class of repressor mutations. Recall that the Lac repressor inhibits transcription of the lac operon in the absence of an inducer but permits transcription when the inducer is present. This regulation is accomplished through a second site on the repressor protein, the allosteric site, which binds to the inducer. When bound to the inducer, the repressor undergoes a change in overall structure such that its DNA-binding site can no longer function.
Jacob and Monod isolated another class of repressor mutation, called superrepressor (IS) mutations. IS mutations cause repression to persist even in the presence of an inducer (compare strain 2 in Table 11-3 with the inducible wild-type strain 1). Unlike I− mutations, IS mutations are dominant over I+ (see Table 11-3, strain 3). This key observation led Jacob and Monod to speculate that IS mutations alter the allosteric site so that it can no longer bind to an inducer. As a consequence, IS-encoded repressor protein continually binds to the operator—preventing transcription of the lac operon even when the inducer is present in the cell. On this basis, we can see why IS is dominant over I+. Mutant IS protein will bind to both operators in the cell, even in the presence of an inducer and regardless of the fact that I+-encoded protein may be present in the same cell (Figure 11-10).
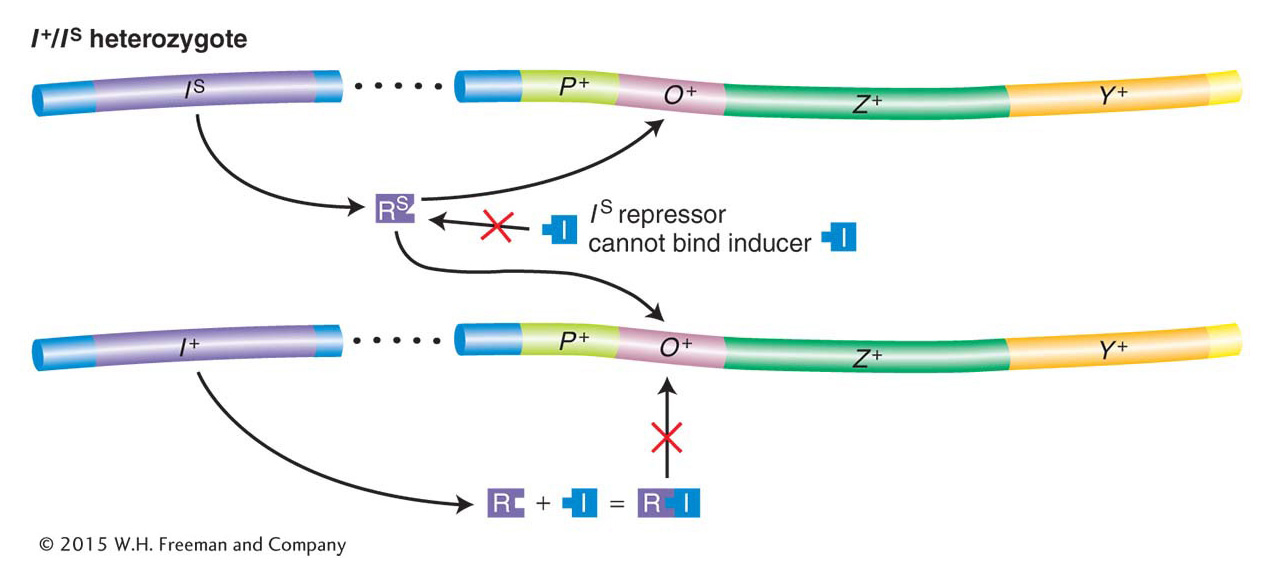
Figure 11-10: The repressor contains a lactose-binding site
Figure 11-10: The dominance of IS mutation is due to the inactivation of the allosteric site on the Lac repressor. In an IS/I− diploid cell, none of the lac structural genes are transcribed. The IS repressor lacks a functional allolactose-binding site (the allosteric site) and thus is not inactivated by an inducer. Therefore, even in the presence of an inducer, the IS repressor binds irreversibly to all operators in a cell, thereby blocking transcription of the lac operon. ANIMATED ART: IS Lac superrepressor mutations
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Note: Bacteria were grown in glycerol (no glucose present) with and without the inducer IPTG. Presence of the indicated enzyme is represented by +; absence or low levels, by −. |
Table 11-3: Synthesis of β-Galactosidase and Permease by the Wild Type and by Strains Carrying Different Alleles of the λ Gene
Genetic analysis of the lac promoter
Mutational analysis also demonstrated that an element essential for lac transcription is located between the gene for the repressor I and the operator site O. This element, termed the promoter (P), serves as the initiation site for transcription by RNA polymerase, as described in Chapter 8. There are two binding regions for RNA polymerase in a typical prokaryotic promoter, shown in Figure 11-11 as the two highly conserved regions at −35 and −10. Promoter mutations are cis-acting in that they affect the transcription of all adjacent structural genes in the operon. Like operators and other cis-acting elements, promoters are sites on the DNA molecule that are bound by proteins and themselves produce no protein product.
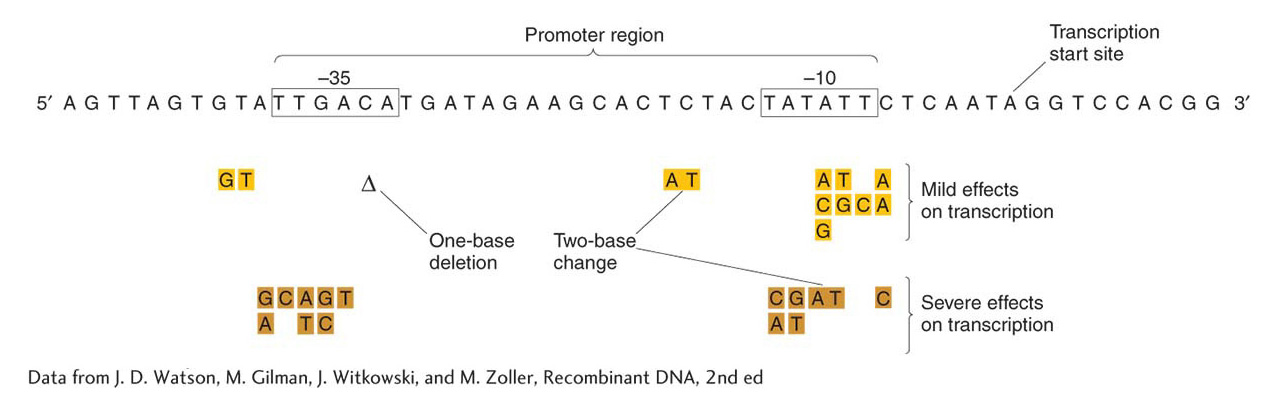
Figure 11-11: RNA polymerase contacts the promoter at specific sequences
Figure 11-11: Specific DNA sequences are important for the efficient transcription of E. coli genes by RNA polymerase. Only the non-template strand is shown here (see Figure 8-4). Transcription would proceed from left to right (5′ to 3′), and the mRNA transcript would be homologous to the sequence shown. The boxed sequences are highly conserved in all E. coli promoters, an indication of their role as contact sites on the DNA for RNA polymerase binding, and contacts are made with both strands (not shown). Mutations in these regions have mild (gold) and severe (brown) effects on transcription. The mutations may be changes of single nucleotides or pairs of nucleotides, or a deletion (Δ) may occur.
[Data from J. D. Watson, M. Gilman, J. Witkowski, and M. Zoller, Recombinant DNA, 2nd ed.]
Molecular characterization of the Lac repressor and the lac operator
Walter Gilbert and Benno Müller-Hill provided a decisive demonstration of the lac system in 1966 by monitoring the binding of the radioactively labeled inducer IPTG to purified repressor protein. They first showed that the repressor consists of four identical subunits, and hence contains four IPTG- (and hence allolactose-) binding sites. Second, they showed that, in the test tube, repressor protein binds to DNA containing the operator and comes off the DNA in the presence of IPTG. (A more detailed description of how the repressor and other DNA-binding proteins work is given later, at the end of Section 11.6.)
Gilbert and his co-workers showed that the repressor can protect specific bases in the operator from chemical reagents. This information allowed them to isolate the DNA segment constituting the operator and to determine its sequence. They took operon DNA to which repressor was bound and treated it with the enzyme DNase, which breaks up DNA. They were able to recover short DNA strands that had been shielded from the enzyme activity by the repressor molecule. These short strands presumably constituted the operator sequence. The base sequence of each strand was determined, and each operator mutation was shown to be a change in the sequence (Figure 11-12). These results showed that the operator locus is a specific sequence of 17 to 25 nucleotides situated just before (5′ to) the structural Z gene. They also showed the incredible specificity of repressor–operator recognition, which can be disrupted by a single base substitution. When the sequence of bases in the lac mRNA (transcribed from the lac operon) was determined, the first 21 bases on the 5′ initiation end proved to be complementary to the operator sequence that Gilbert had determined, showing that the operator sequence is transcribed.
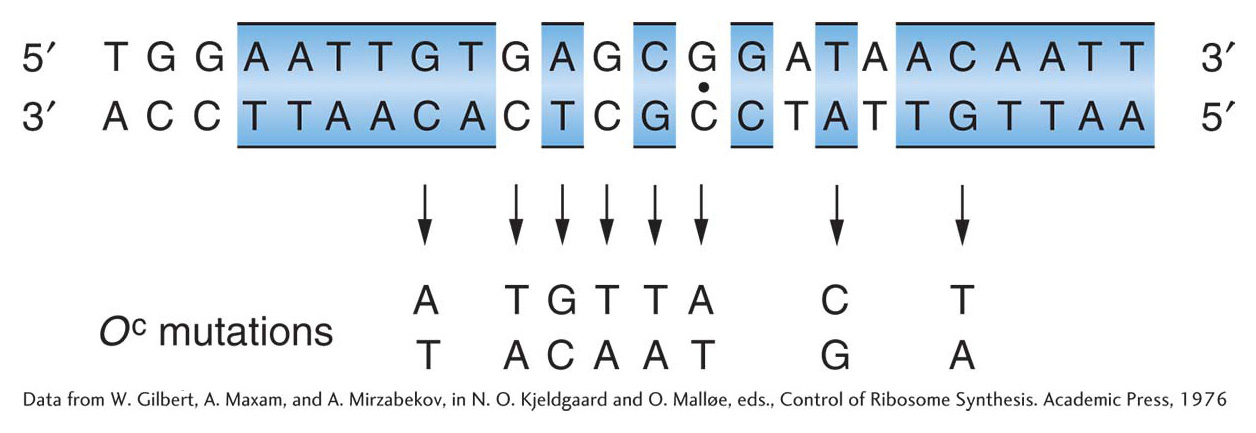
Figure 11-12: The operator is a specific DNA sequence
Figure 11-12: The DNA base sequence of the lactose operator and the base changes associated with eight OC mutations. Regions of twofold rotational symmetry are indicated by color and by a dot at their axis of symmetry.
[Data from W. Gilbert, A. Maxam, and A. Mirzabekov, in N. O. Kjeldgaard and O. Mall0e, eds., Control of Ribosome Synthesis. Academic Press, 1976]
The results of these experiments provided crucial confirmation of the mechanism of repressor action formulated by Jacob and Monod.





