11.5 Metabolic Pathways and Additional Levels of Regulation: Attenuation
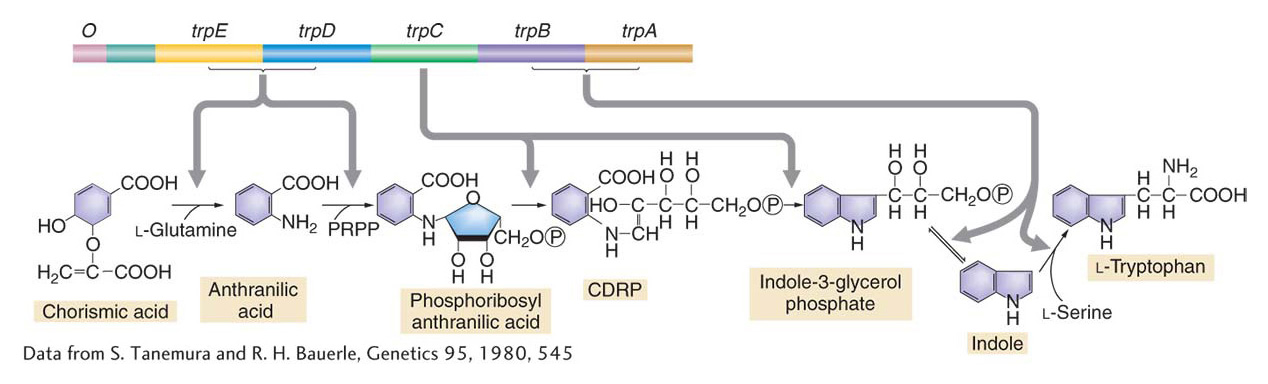
Coordinate control of genes in bacteria is widespread. In the preceding section, we looked at examples illustrating the regulation of pathways for the breakdown of specific sugars. In fact, most coordinated genes in bacteria are coordinated through operon mechanisms. In many pathways that synthesize essential molecules from simple inorganic building blocks, the genes that encode the enzymes are organized into operons, complete with multigenic mRNAs. Furthermore, in cases for which the sequence of catalytic activity is known, there is a remarkable congruence between the order of operon genes on the chromosome and the order in which their products act in the metabolic pathway. This congruence is strikingly illustrated by the organization of the tryptophan operon in E. coli (Figure 11-21). The tryptophan operon contains five genes (trpE, trpD, trpC, trpB, trpA) that encode enzymes that contribute to the synthesis of the amino acid tryptophan.
KEY CONCEPT
In bacteria, genes that encode enzymes that are in the same metabolic pathways are generally organized into operons.There are two mechanisms for regulating transcription of the tryptophan operon and some other operons functioning in amino acid biosynthesis. One provides global control of operon mRNA expression, and the other provides fine-
The level of trp operon gene expression is governed by the level of tryptophan. When tryptophan is absent from the growth medium, trp gene expression is high; when levels of tryptophan are high, the trp operon is repressed. One mechanism for controlling the transcription of the trp operon is similar to the mechanism that we have already seen controls the lac operon: a repressor protein binds an operator, preventing the initiation of transcription. This repressor is the Trp repressor, the product of the trpR gene. The Trp repressor binds tryptophan when adequate levels of the amino acid are present, and only after binding tryptophan will the Trp repressor bind to the operator and switch off transcription of the operon. This simple mechanism ensures that the cell does not waste energy producing tryptophan when the amino acid is sufficiently abundant. E. coli strains with mutations in trpR continue to express the trp mRNA and thus continue to produce tryptophan when the amino acid is abundant.
415
In studying these trpR mutant strains, Charles Yanofsky discovered that, when tryptophan was removed from the medium, the production of trp mRNA further increased several-
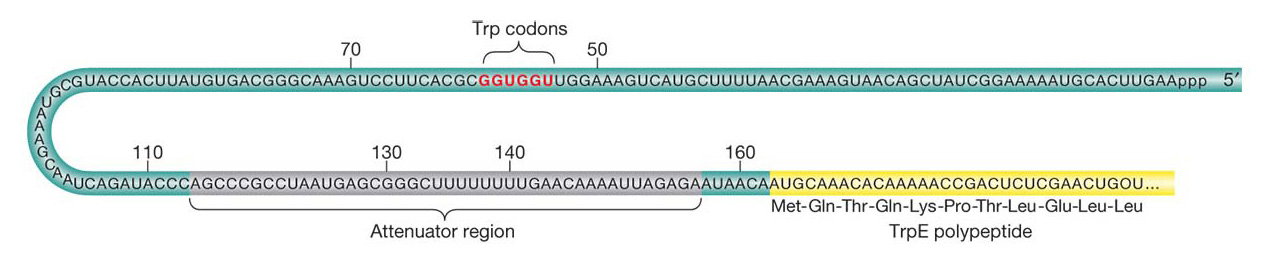
The mechanisms governing attenuation were discovered by identifying mutations that reduced or abolished attenuation. Strains with these mutations produce trp mRNA at maximal levels even in the presence of tryptophan. Yanofsky mapped the mutations to a region between the trp operator and the trpE gene; this region, termed the leader sequence, is at the 5′ end of the trp operon mRNA before the first codon of the trpE gene (Figure 11-22). The trp leader sequence is unusually long for a prokaryotic mRNA, 160 bases, and detailed analyses have revealed how a part of this sequence works as an attenuator that governs the further transcription of trp mRNA.
416
The key observations are that, in the absence of the TrpR repressor protein, the presence of tryptophan halts transcription after the first 140 bases or so, whereas, in the absence of tryptophan, transcription of the operon continues. The mechanism for terminating or continuing transcription consists of two key elements. First, the trp mRNA leader sequence encodes a short, 14-
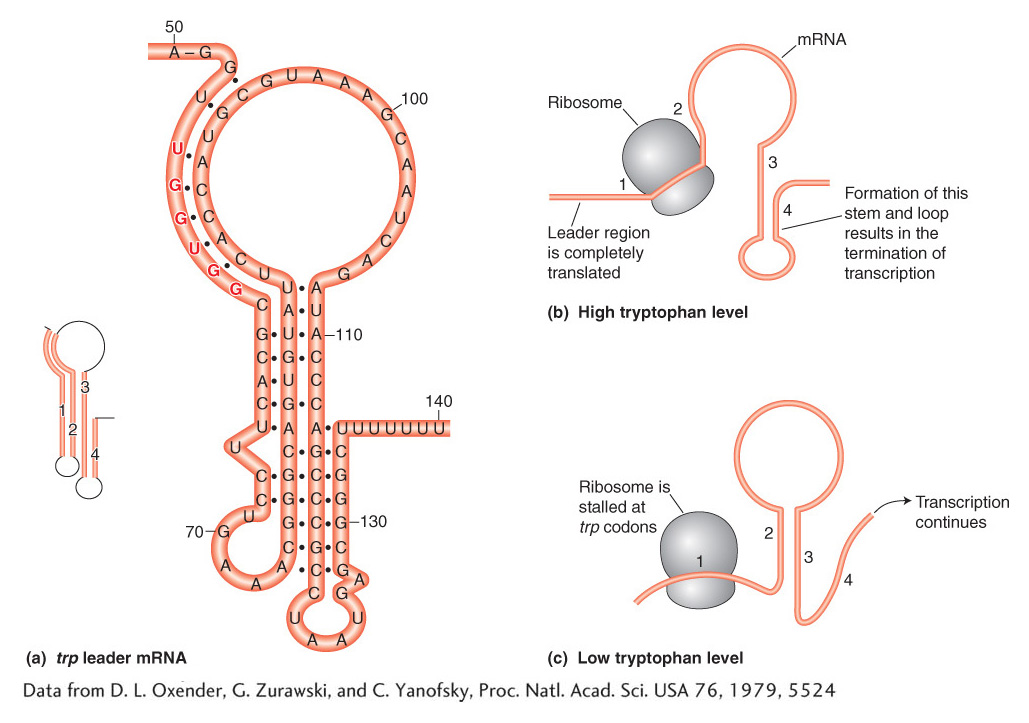
The regulatory logic of the operon pivots on the abundance of tryptophan. When tryptophan is abundant, there is a sufficient supply of aminoacyl-
Other operons for enzymes in biosynthetic pathways have similar attenuation controls. One signature of amino acid biosynthesis operons is the presence of multiple codons for the amino acid being synthesized in a separate peptide encoded by the 5′ leader sequence. For instance, the phe operon has seven phenylalanine codons in a leader peptide and the his operon has seven tandem histidine codons in its leader peptide (Figure 11-24).

417