11.6 Bacteriophage Life Cycles: More Regulators, Complex Operons
In that Paris movie theater, François Jacob had a flash of insight that the phenomenon of prophage induction might be closely analogous to the induction of β-galactosidase synthesis. He was right. Here, we are going to see how the life cycle of the bacteriophage λ is regulated. Although its regulation is more complex than that of individual operons, it is controlled by now-familiar modes of gene regulation.
Bacteriophage λ is a so-called temperate phage that has two alternative life cycles (Figure 11-25). When a normal bacterium is infected by a wild-type λ phage, two possible outcomes may follow: (1) the phage may replicate and eventually lyse the cell (the lytic cycle) or (2) the phage genome may be integrated into the bacterial chromosome as an inert prophage (the lysogenic cycle). In the lytic state, most of the phage’s 71 genes are expressed at some point, whereas in the lysogenic state, most genes are inactive.
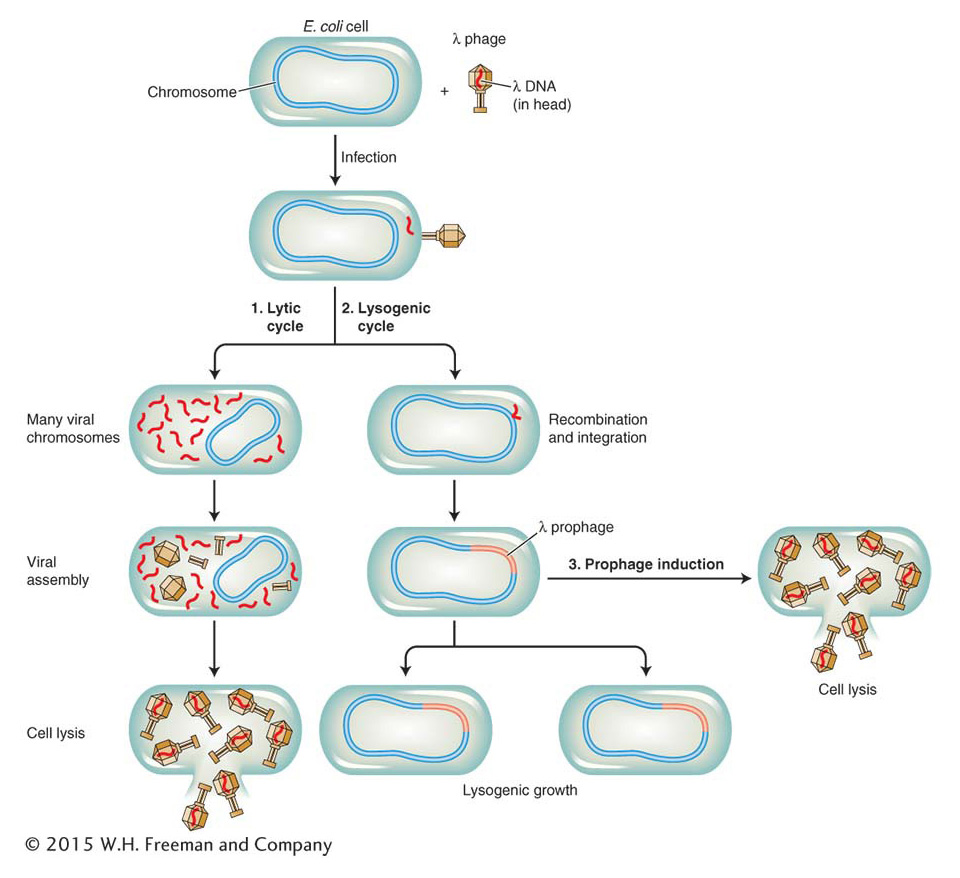
Figure 11-25: The life cycle of bacteriophage λ
Figure 11-25: Whether bacteriophage λ enters the lytic cycle immediately or enters the lysogenic cycle depends on the availability of resources. The lysogenic virus inserts its genome into the bacterial chromosome, where it remains quiescent until conditions are favorable.
What decides which of these two pathways is taken? The physiological control of the decision between the lytic or lysogenic pathway depends on the resources available in the host bacterium. If resources are abundant, the lytic cycle is preferred because then there are sufficient nutrients to make many copies of the virus. If resources are limited, the lysogenic pathway is taken. The virus then remains present as a prophage until conditions improve. The inert prophage can be induced by ultraviolet light to enter the lytic cycle—the phenomenon studied by Jacob. The lytic and lysogenic states are characterized by very distinct programs of gene expression that must be regulated. Which alternative state is selected is determined by a complex genetic switch comprising several DNA-binding regulatory proteins and a set of operator sites.
Just as they were for the lac and other regulatory systems, genetic analyses of mutants were sources of crucial insights into the components and logic of the λ genetic switch. Jacob used simple phenotypic screens to isolate mutants that were defective in either the lytic or the lysogenic pathway. Mutants of each type could be recognized by the appearance of infected plaques on a lawn of bacteria. When wild-type phage particles are placed on a lawn of sensitive bacteria, clearings (called “plaques”) appear where bacteria are infected and lysed, but these plaques are turbid because bacteria that are lysogenized grow within them (Figure 11-26). Mutant phages that form clear plaques are unable to lysogenize cells.
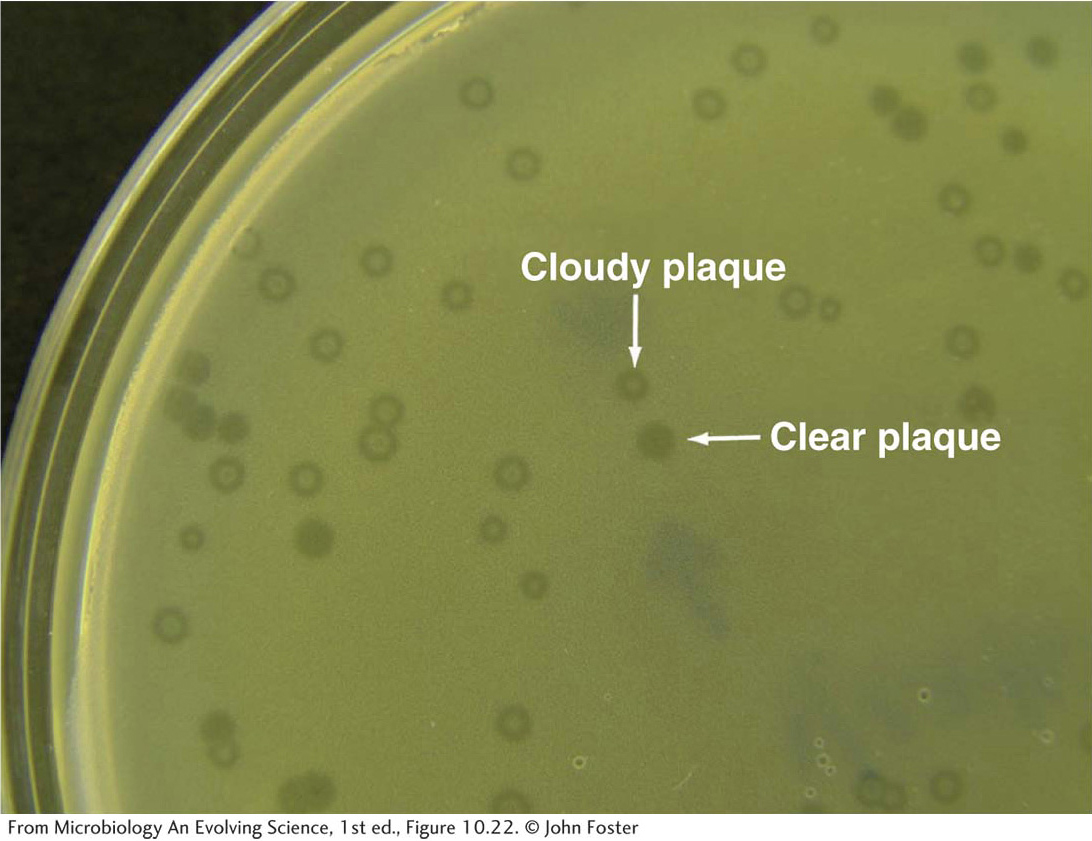
Figure 11-26: Clear and cloudy bacteriophage plaques on a lawn of E. coli host bacteria
Figure 11-26: Plaques are clear where host cell lysis has occurred; they are cloudy where cells have survived infection and continued to grow as a lysogen.
[From Microbiology An Evolving Science, 1st ed., Figure 10.22. © John Foster]
Such clear mutants (designated by c) turn out to be analogous to the I and O mutants of the lac system. These mutants were often isolated as temperature-sensitive mutants that had clear phenotypes at higher temperatures but wild-type phenotypes at lower temperatures. Three classes of mutants led to the identification of the key regulatory features of phage λ. In the first class, mutants for the cI, cII, and cIII genes form clear plaques; that is, they are unable to establish lysogeny. A second class of mutants were isolated that do not lysogenize cells but can replicate and enter the lytic cycle in a lysogenized cell. These mutants turn out to be analogous to the operator-constitutive mutants of the lac system. A third key mutant can lysogenize but is unable to lyse cells. The mutated gene in this case is the cro gene (for control of repressor and other things). The decision between the lytic and the lysogenic pathways hinges on the activity of the proteins encoded by the four genes cI, cII, cIII, and cro, three of which are DNA-binding proteins.
We will first focus on the two genes cI and cro and the proteins that they encode (Table 11-4). The cI gene encodes a repressor, often referred to as λ repressor, that represses lytic growth and promotes lysogeny. The cro gene encodes a repressor that represses lysogeny, thereby permitting lytic growth. The genetic switch controlling the two λ phage life cycles has two states: in the lysogenic state, cI is on, but cro is off, and in the lytic cycle, cro is on, but cI is off. Therefore, λ repressor and Cro are in competition, and whichever repressor prevails determines the state of the switch and of the expression of the λ genome.
Table 11-4: Major Regulators of Bacteriophage λ Life Cycle
The race between λ repressor and Cro is initiated when phage λ infects a normal bacterium. The sequence of events in the race is critically determined by the organization of genes in the λ genome and of promoters and operators between the cI and the cro genes. The roughly 50-kb λ genome encodes proteins having roles in DNA replication, recombination, assembly of the phage particle, and cell lysis (Figure 11-27). These proteins are expressed in a logical sequence such that copies of the genome are made first, these copies are then packaged into viral particles, and, finally, the host cell is lysed to release the virus and begin the infection of other host cells (see Figure 11-25). The order of viral gene expression flows from the initiation of transcription at two promoters, PL and PR (for leftward and rightward promoter with respect to the genetic map). On infection, RNA polymerase initiates transcription at both promoters. Looking at the genetic map (see Figure 11-27), we see that from PR, cro is the first gene transcribed, and from PL, N is the first gene transcribed.
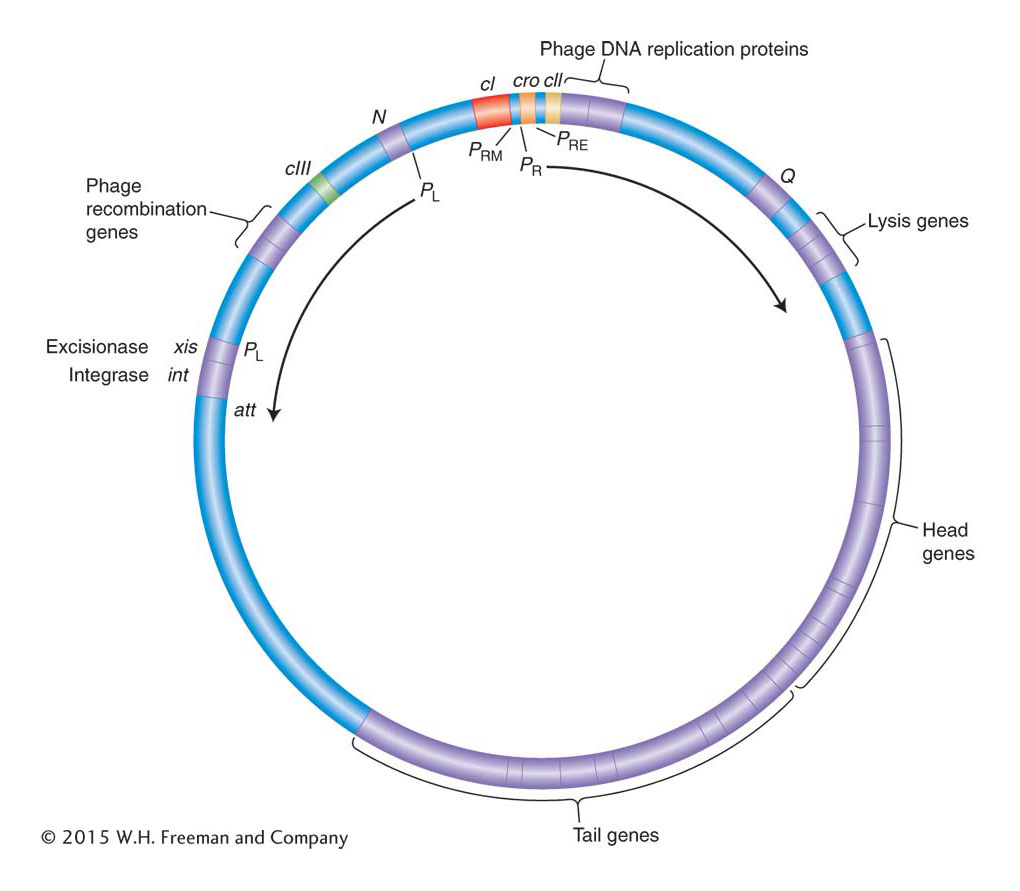
Figure 11-27: The phage λ genome organization facilitates coordinate control
Figure 11-27: Map of phage λ in the circular form. The genes for recombination, integration and excision, replication, head and tail assembly, and cell lysis are clustered together and coordinately regulated. Transcription of the right side of the genome begins at PR, and that of the leftward genes begins at PL. Key regulatory interactions governing the lysogenic-versus-lytic decision take place at operators between the cro and the cI genes.
The N gene encodes a positive regulator, but the mechanism of this protein differs from those of other regulators that we have considered thus far. Protein N works by enabling RNA polymerase to continue to transcribe through regions of DNA that would otherwise cause transcription to terminate. A regulatory protein such as N that acts by preventing transcription termination is called an antiterminator. Thus, N allows the transcription of cIII and other genes to the left of N, as well as cII and other genes to the right of cro. The cII gene encodes an activator protein that binds to a site that promotes transcription leftward from a different promoter, PRE (for promoter of repressor establishment), which activates transcription of the cI gene. Recall that the cI gene encodes λ repressor, which will prevent lytic growth.
Before the expression of the rest of the viral genes takes place, a “decision” must be made—whether to continue with viral-gene expression and lyse the cell or to repress that pathway and lysogenize the cell. The decision whether to lyse or lysogenize a cell pivots on the activity of the cII protein. The cII protein is unstable because it is sensitive to bacterial proteases—enzymes that degrade proteins. These proteases respond to environmental conditions: they are more active when resources are abundant but less active when cells are starved.
Let’s look at what happens to cII when resources are abundant and not abundant. When resources are abundant, cII is degraded and little λ repressor is produced. The genes transcribed from PL and PR continue to be expressed, and the lytic cycle prevails. However, if resources are limited, cII is more active and more λ repressor is produced. In this case, the genes transcribed from PL and PR are repressed by the λ repressor and the lysogenic cycle is entered. The cII protein is also responsible for activating the transcription of int, a gene that encodes an additional protein required for lysogeny—an integrase required for the λ genome to integrate into the host chromosome. The cIII protein shields cII from degradation; so it, too, contributes to the lysogenic decision.
Let’s briefly recap the sequence of events and the decision points in the bacteriophage λ life cycle:
Host RNA polymerase initiates transcription at PL and PR, expressing the cro and N genes.
Antiterminator protein N enables transcription of the cIII gene and recombination genes (see Figure 11-27, left), and the cII gene and other genes (see Figure 11-27, right).
The cII protein, protected by the cIII protein, turns on cI and int by activating transcription at PRE.
If resources and proteases are abundant, cII is degraded, Cro represses cI, and the lytic cycle continues.
If resources and proteases are not abundant, cII is active, cI transcription proceeds at a high level, Int protein integrates the phage chromosome, and cI (λ repressor) shuts off all genes except itself.
Molecular anatomy of the genetic switch
To see how the decision is executed at the molecular level, let’s turn to the activities of λ repressor and Cro. The OR operator lies between the two genes encoding these proteins and contains three sites, OR1, OR2, and OR3, that overlap two opposing promoters: PR, which promotes transcription of lytic genes, and PRM (for repressor maintenance), which directs transcription of the cI gene (see Figure 11-27). Recall that the cI gene encodes the λ repressor. The three operator sites are similar but not identical in sequence, and although Cro and λ repressor can each bind to any one of the operators, they do so with different affinities: λ repressor binds to OR1 with the highest affinity, whereas Cro binds to OR3 with the highest affinity. The λ repressor’s occupation of OR1 blocks transcription from PR and thus blocks the transcription of genes for the lytic cycle. Cro’s occupation of OR3 blocks transcription from PRM and thus blocks maintenance of cI transcription. Hence, no λ repressor is produced, and transcription of genes for the lytic cycle can continue. The occupation of the operator sites therefore determines the lytic-versuslysogenic patterns of λ gene expression (Figure 11-28).
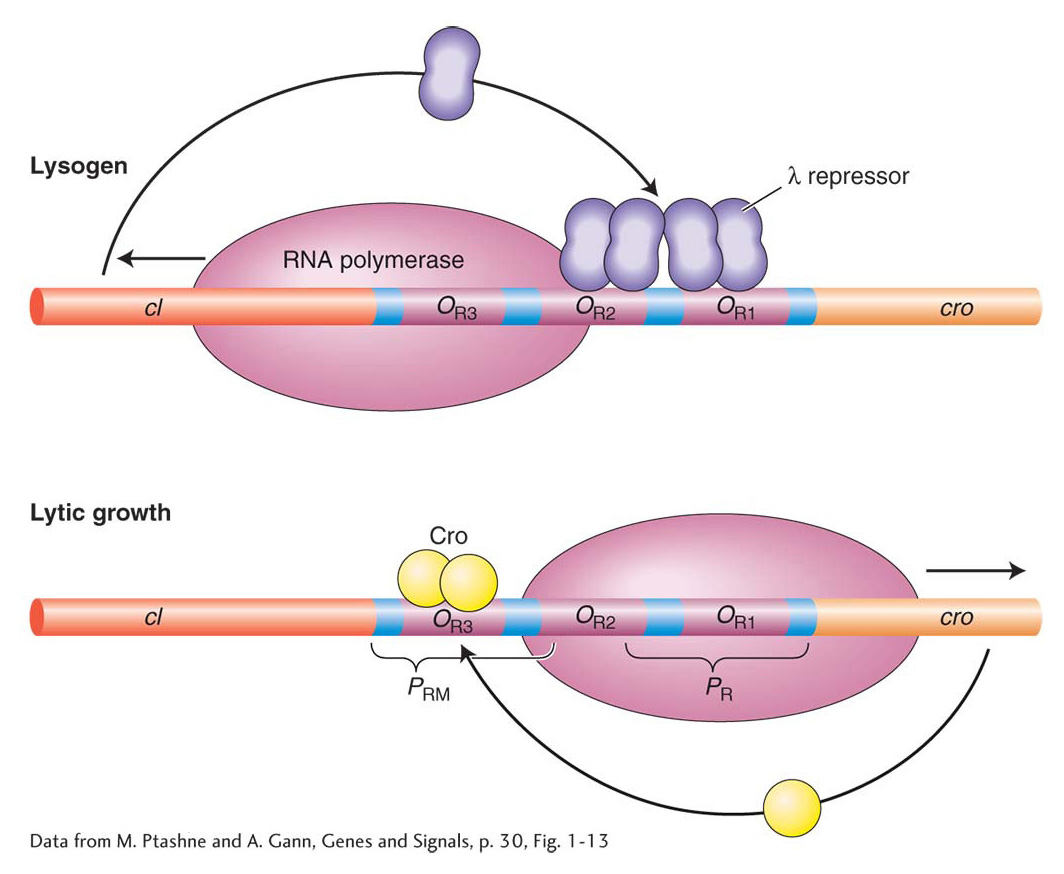
Figure 11-28: The lysogenic-versus-lytic cycle is determined by repressor occupancy on the OR operators
Figure 11-28: The binding of λ repressor and Cro to operator sites. Lysogeny is promoted by λ repressor binding to OR1 and OR2, which prevents transcription from PR. On induction or in the lytic cycle, the binding of Cro to OR3 prevents transcription of the cI gene.
[Data from M. Ptashne and A. Gann, Genes and Signals, p. 30, Fig. 1-13]
After a lysogen has been established, it is generally stable. But the lysogen can be induced to enter the lytic cycle by various environmental changes. Ultraviolet light induces the expression of host genes. One of the host genes encodes a protein, RecA, that stimulates cleavage of the λ repressor, thus crippling maintenance of lysogeny and resulting in lytic growth. Prophage induction, just as Jacob and Monod surmised, requires the release of a repressor from DNA. The physiological role of ultraviolet light in lysogen induction makes sense in that this type of radiation damages host DNA and stresses the bacteria; the phage replicates and leaves the damaged, stressed cell for another host.
KEY CONCEPT
The phage λ genetic switch illustrates how a few DNA-binding regulatory proteins, acting through a few sites, control the expression of a much larger number of genes in the virus in a “cascade” mechanism. Just as in the lac, ara, trp, and other systems, the alternative states of gene expression are determined by physiological signals.
Sequence-specific binding of regulatory proteins to DNA
How do λ repressor and Cro recognize different operators with different affinities? This question directs our attention to a fundamental principle in the control of gene transcription—the regulatory proteins bind to specific DNA sequences. For individual proteins to bind to certain sequences and not others requires specificity in the interactions between the side chains of the protein’s amino acids and the chemical groups of DNA bases. Detailed structural studies of λ repressor, Cro, and other bacterial regulators have revealed how the three-dimensional structures of regulators and DNA interact and how the arrangement of particular amino acids enables them to recognize specific base sequences.
Crystallographic analysis has identified a common structural feature of the DNA-binding domains of λ and Cro. Both proteins make contact with DNA through a helix-turn-helix domain that consists of two α helices joined by a short flexible linker region (Figure 11-29). One helix, the recognition helix, fits into the major groove of DNA. In that position, amino acids on the helix’s outer face are able to interact with chemical groups on the DNA bases. The specific amino acids in the recognition helix determine the affinity of a protein for a specific DNA sequence.
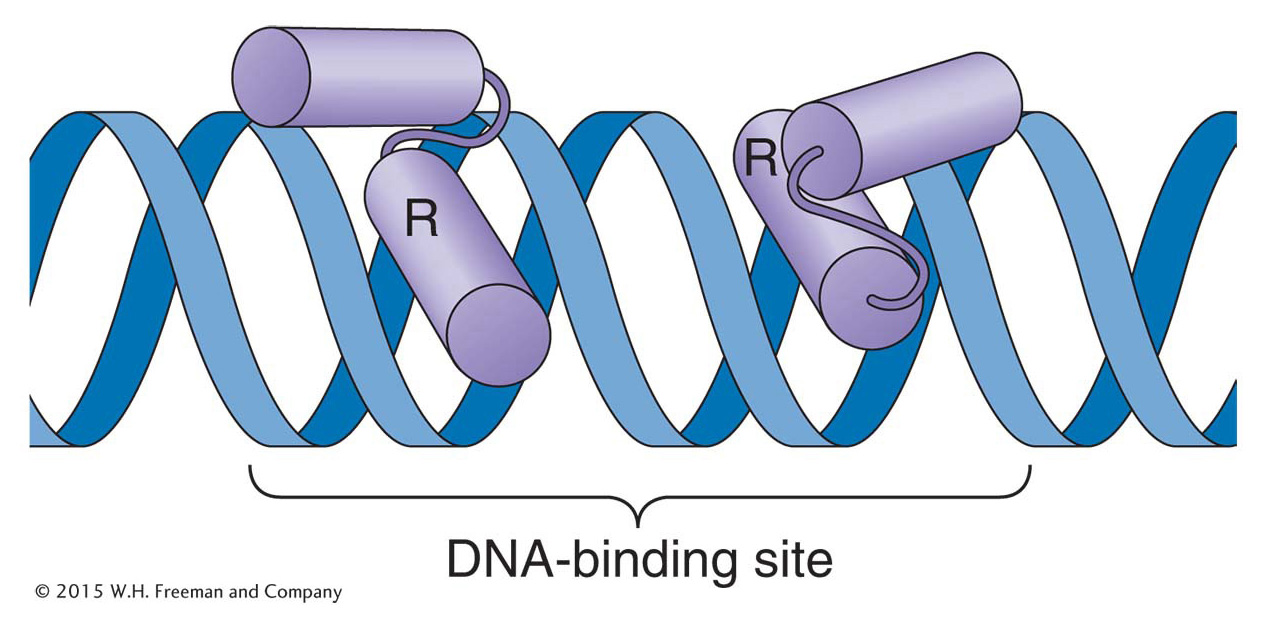
Figure 11-29: Helix-turn-helix is a common DNA-binding motif
Figure 11-29: The binding of a helix-turn-helix motif to DNA. The purple cylinders are alpha helices. Many regulatory proteins bind as dimers to DNA. In each monomer, the recognition helix (R) makes contact with bases in the major groove of DNA.
The recognition helices of the λ repressor and Cro have similar structures and some identical amino acid residues. Differences between the helices in key amino acid residues determine their DNA-binding properties. For example, in the λ repressor and Cro proteins, glutamine and serine side chains contact the same bases, but an alanine residue in the λ repressor and lysine and asparagine residues in the Cro protein impart different binding affinities for sequences in OR1 and OR3 (Figure 11-30).
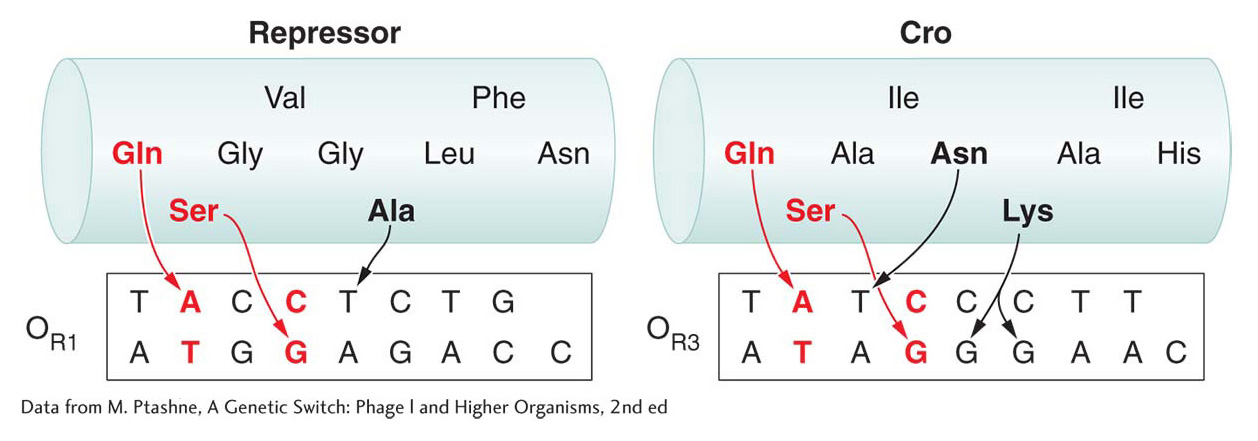
Figure 11-30: Amino acid side chains determine the specificity of DNA binding
Figure 11-30: Interactions between amino acids and bases determine the specificity and affinity of DNA-binding proteins. The amino acid sequences of the recognition helices of the λ repressor and Cro proteins are shown. Interactions between the glutamine (Gln), serine (Ser), and alanine (Ala) residues of the λ repressor and bases in the OR operator determine the strength of binding. Similarly, interactions between the glutamine, serine, asparagine (Asn), and lysine (Lys) residues of the Cro protein mediate binding to the OR3 operator. Each DNA sequence shown is that bound by an individual monomer of the respective repressor; it is half of the operator site occupied by the repressor dimer.
[Data from M. Ptashne, A Genetic Switch: Phage l and Higher Organisms, 2nd ed.]
The Lac and TrpR repressors, as well as the AraC activator and many other proteins, also bind to DNA through helix-turn-helix motifs of differing specificities, depending on the primary amino acid sequences of their recognition helices. In general, other domains of these proteins, such as those that bind their respective allosteric effectors, are dissimilar.
KEY CONCEPT
The biological specificity of gene regulation is due to the chemical specificity of amino acid-base interactions between individual regulatory proteins and discrete DNA sequences.





