12.1 Transcriptional Regulation in Eukaryotes: An Overview
The biological properties of each eukaryotic cell type are largely determined by the proteins expressed within it. This constellation of expressed proteins determines much of the cell’s architecture, its enzymatic activities, its interactions with its environment, and many other physiological properties. However, at any given time in a cell’s life history, only a fraction of the RNAs and proteins encoded in its genome are expressed. At different times, the profile of expressed gene products can differ dramatically, both in regard to which proteins are expressed and at what levels. How are these specific profiles generated?
As one might expect, if the final product is a protein, regulation could be achieved by controlling the transcription of DNA into RNA or the translation of RNA into protein. In fact, gene regulation takes place at many levels, including at the mRNA level (through alterations in splicing or the stability of the mRNA) and after translation (by modifications of proteins). The varied ways that eukaryotic genes can be regulated have been divided into two general categories that reflect when they act: transcriptional gene regulation and post-transcriptional gene regulation. While the former will be the primary focus of this chapter, the latter is receiving increasing attention. In particular, the role of RNA to act post-transcriptionally to repress gene expression (called gene silencing; see Chapter 8) is one of the hottest areas of current research. Three of the RNA players that participate in silencing genes, miRNA, ncRNA, and siRNA, were introduced in Chapter 8. Later in this chapter we will explore the mechanisms of gene regulation mediated by miRNA and ncRNA.
Most regulation characterized to date takes place at the level of gene transcription; so, in this chapter, the primary focus is on transcriptional gene regulation. The basic mechanism at work is that molecular signals from outside or inside the cell lead to the binding of regulatory proteins to specific DNA sites outside of protein-encoding regions, and the binding of these proteins modulates the rate of transcription. These proteins may directly or indirectly assist RNA polymerase in binding to its transcription initiation site—the promoter—or they may repress transcription by preventing the binding of RNA polymerase.
Although bacteria and eukaryotes have much of the logic of gene regulation in common, there are some fundamental differences in the underlying mechanisms and machinery. Both use sequence-specific DNA-binding proteins to modulate the level of transcription. However, eukaryotic genomes are bigger, and their range of properties is larger than that of bacteria. Inevitably, the regulation of eukaryotic genomes is more complex, reflecting their structural and functional complexity. This requires more types of regulatory proteins and more types of interactions with the adjacent regulatory regions in DNA. One notable difference is that eukaryotic DNA is packaged into nucleosomes, forming chromatin, whereas bacterial DNA lacks nucleosomes. In eukaryotes, chromatin structure is dynamic and is an essential ingredient in gene regulation.
In general, the ground state of a bacterial gene is “on.” Thus, RNA polymerase can usually bind directly to a promoter when no other regulatory proteins are around to bind to the DNA. In bacteria, transcription initiation is prevented or reduced if the binding of RNA polymerase is blocked, usually through the binding of a repressor regulatory protein. Activator regulatory proteins increase the binding of RNA polymerase to promoters where a little help is needed. In contrast, the ground state in eukaryotes is “off.” Therefore, the transcriptional machinery (including RNA polymerase II and associated general transcription factors) cannot bind to the promoter in the absence of other regulatory proteins (Figure 12-2). In many cases, the binding of the transcriptional apparatus is not possible because nucleosomes are positioned to block the promoter. Thus, chromatin structure usually has to be changed to activate eukaryotic transcription. Those changes generally depend on the binding of sequence-specific DNA-binding regulatory proteins. The structure of chromatin around activated or repressed genes within cells can be quite stable and inherited by daughter cells. The inheritance of chromatin states is a form of inheritance that does not directly entail DNA sequence; it provides a means of epigenetic regulation.
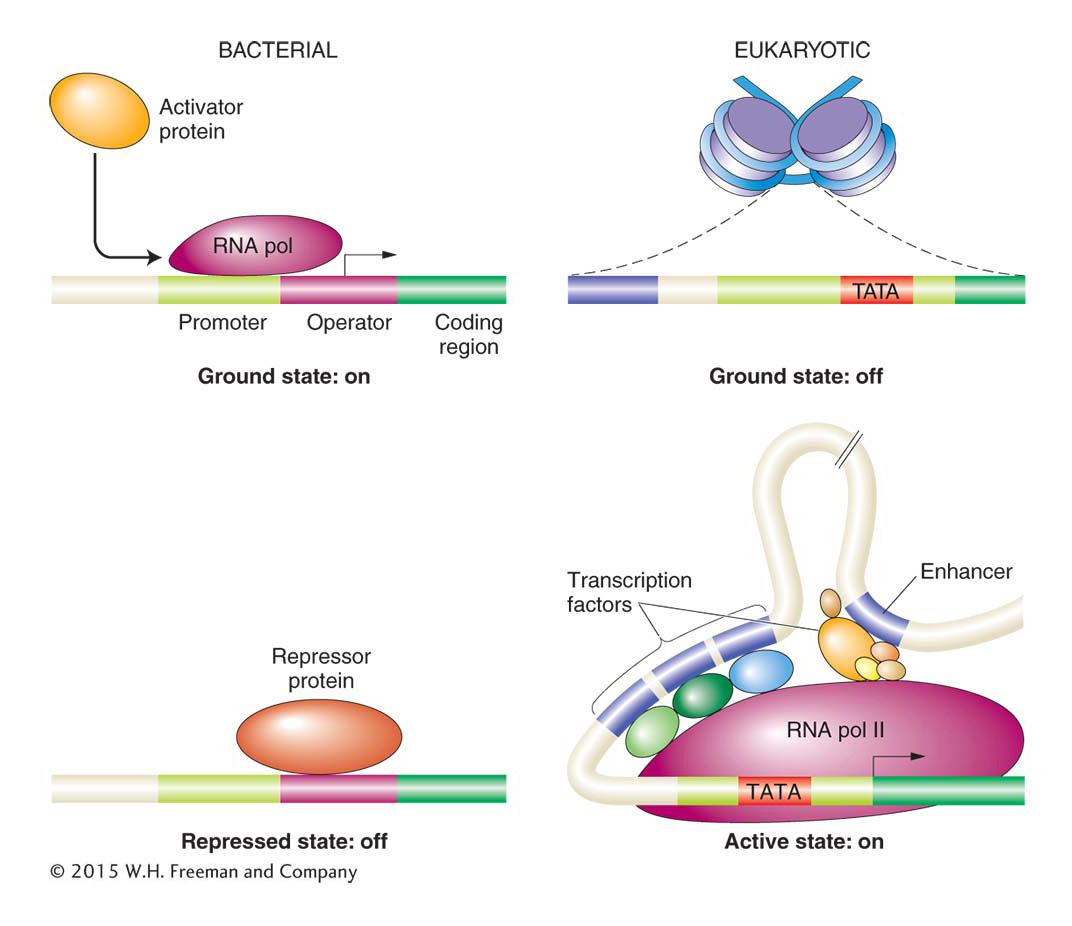
Figure 12-2: Overview of transcriptional regulation
Figure 12-2: In bacteria, RNA polymerase can usually begin transcription unless a repressor protein blocks it. In eukaryotes, however, the packaging of DNA with nucleosomes prevents transcription unless other regulatory proteins are present. These regulatory proteins expose promoter sequences by altering nucleosome density or position. They may also recruit RNA polymerase II more directly through binding.
The unique features of eukaryotic transcriptional regulation are the focus of this chapter. Some differences from bacteria in the process of transcription and that affect regulation were already noted in Chapter 8:
In bacteria, all genes are transcribed into RNA by the same RNA polymerase, whereas three RNA polymerases function in eukaryotes. RNA polymerase II, which transcribes DNA into mRNA, was the focus of Chapter 8 and will be the only polymerase discussed in this chapter.
RNA transcripts are extensively processed during transcription in eukaryotes; the 5′ and 3′ ends are modified and introns are spliced out.
RNA polymerase II is much larger and more complex than its bacterial counterpart. One reason it is more complex is that RNA polymerase II must synthesize RNA and coordinate the special processing events unique to eukaryotes.
Multicellular eukaryotes may have as many as 25,000 genes, several-fold more than the average bacterium. Moreover, patterns of eukaryotic gene expression can be extraordinarily complex. That is, the timing of gene expression, and the amount of transcript produced, vary widely among eukaryotic genes. For example, one gene may be transcribed only during one stage of development and another only in the presence of a viral infection. Finally, the majority of the genes in a eukaryotic cell are off at any one time. On the basis of these considerations alone, eukaryotic gene regulation must be able to
ensure that the expression of most genes in the genome is off at any one time while activating a subset of genes.
generate thousands of patterns of gene expression.
As you will see later in the chapter, mechanisms have evolved to ensure that most of the genes in a eukaryotic cell are not transcribed. Before considering how genes are kept transcriptionally inactive, we will focus on the second point: How are eukaryotic genes able to exhibit an enormous number and diversity of expression patterns? The machinery required for generating so many patterns of gene transcription in vivo has many components, including both trans-acting regulatory proteins and cis-acting regulatory DNA sequences. We can divide the regulatory proteins into two sets based on the DNA regulatory sequences they bind. The first set of proteins are the large RNA polymerase II complex and the general transcription factors that you learned about in Chapter 8. To initiate transcription, these proteins interact with cis-acting regulatory DNA sequences called promoter-proximal elements near the promoter of a gene. The second set of regulatory proteins consists of transcription factors that bind to cis-acting regulatory DNA sequences called enhancers. These regulatory sequences may be located a considerable distance from gene promoters. Generally speaking, promoters and promoter-proximal elements are bound by transcription factors that affect the expression of many genes. Enhancers are bound by transcription factors that control the regulation of smaller subsets of genes. Often, an enhancer will act in only one or a few cell types in a multicellular eukaryote. Much of the strategy of eukaryotic transcriptional control hinges on how specific transcription factors control the access of general transcription factors and RNA polymerase II.
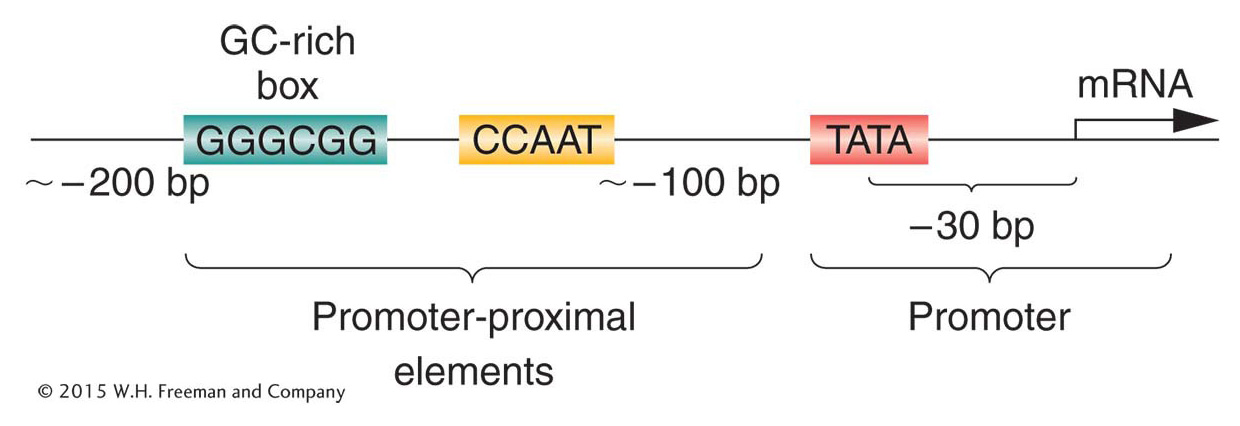
Figure 12-3: Promoter-proximal elements precede the promoter of a eukaryotic gene
Figure 12-3: The region upstream of the transcription start site in higher eukaryotes contains promoter-proximal elements and the promoter.
For RNA polymerase II to transcribe DNA into RNA at a maximum rate, multiple cis-acting regulatory elements must play a part. The promoters, promoter-proximal elements, and enhancers are all targets for binding by different trans-acting DNA-binding proteins. Figure 12-3 is a schematic representation of the promoter and promoter-proximal sequence elements. The binding of RNA polymerase II to the promoter does not produce efficient transcription by itself. Transcription requires the binding of general transcription factors to additional promoter-proximal elements that are commonly found within 100 bp of the transcription initiation site of many (but not all) genes. One of these elements is the CCAAT (pronounced “cat”) box, and often another is a GC-rich segment farther upstream. The general transcription factors that bind to the promoter-proximal elements are expressed in most cells, and so they are available to initiate transcription at any time. Mutations in these sites can have a dramatic effect on transcription, demonstrating how important they are. If these sequence elements are mutated, the level of transcription is generally reduced, as shown in Figure 12-4.
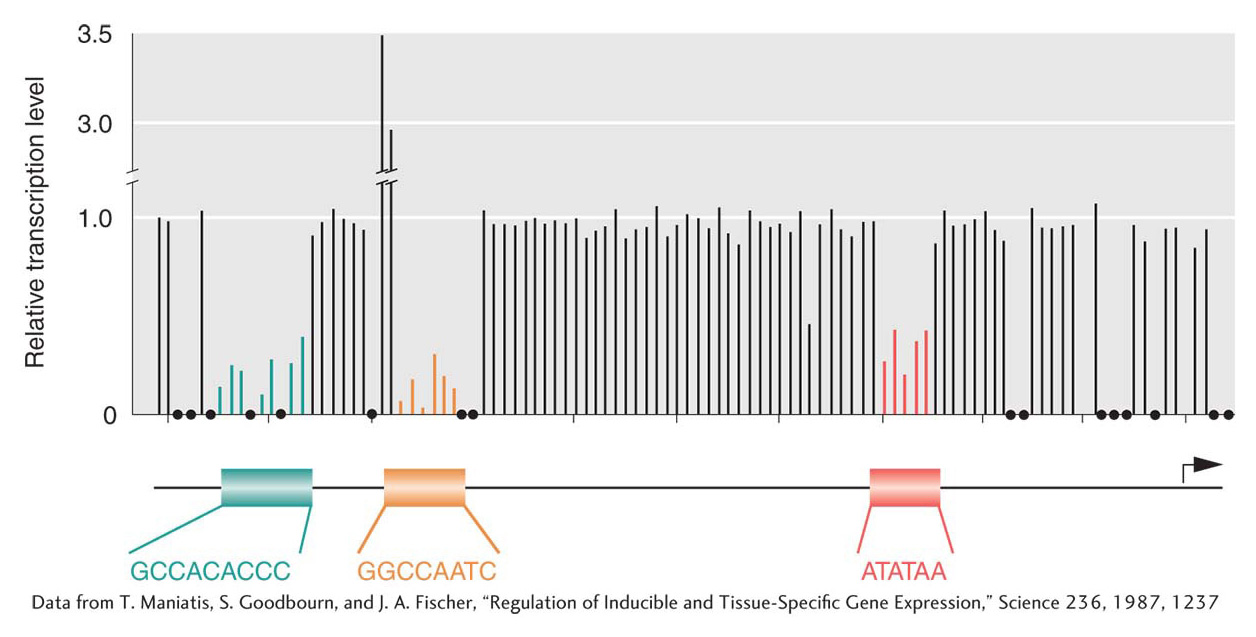
Figure 12-4: Promoter-proximal elements are necessary for efficient transcription
Figure 12-4: Point mutations in the promoter and promoter-proximal elements hinder transcription of the β-globin gene. Point mutations throughout the promoter region were analyzed for their effects on transcription rates. The height of each line represents the transcription level relative to a wild-type promoter or promoter-proximal element (1.0). Only the base substitutions that lie within the three elements shown change the level of transcription. Positions with black dots were not tested.
[Data from T. Maniatis, S. Goodbourn, and J. A. Fischer, “Regulation of Inducible and Tissue-Specific Gene Expression,” Science 236, 1987, 1237]
To modulate transcription, regulatory proteins possess one or more of the following functional domains:
A domain that recognizes a DNA regulatory sequence (the protein’s DNA-binding site)
A domain that interacts with one or more proteins of the transcriptional apparatus (RNA polymerase or a protein associated with RNA polymerase)
A domain that interacts with proteins bound to nearby regulatory sequences on DNA such that they can act cooperatively to regulate transcription
A domain that influences chromatin condensation either directly or indirectly
A domain that acts as a sensor of physiological conditions within the cell
Eukaryotic gene regulatory mechanisms have been discovered through both biochemical and genetic approaches. The latter has been advanced in particular by studies of the single-celled yeast Saccharomyces cerevisiae (see the Model Organism box). This organism, which has played a key role in wine-making, beermaking, and baking for many centuries has been a passport to understanding much of eukaryotic cell biology. Several decades of research have produced many fundamental insights into general principles of how eukaryotic transcriptional regulatory proteins work and how different cell types are generated. We’ll examine two yeast gene regulatory systems in detail: the first concerns the galactose-utilization pathway; the second is the control of mating type.


