12.6 Gender-Specific Silencing of Genes and Whole Chromosomes
Thus far, we have discussed chromosomal domains that are open or condensed in all members of a species. In this section we consider two widespread genetic phenomena in mammals that depend on the sex of the individual. In these cases, specific genes or even a whole chromosome are silenced for the entire lifetime of an organism. However, unlike the prior examples, these genes or chromosomes are silenced in males or females but not both.
Genomic imprinting explains some unusual patterns of inheritance
The phenomenon of genomic imprinting was discovered about 20 years ago in mammals. In genomic imprinting, certain autosomal genes have unusual inheritance patterns. For example, an Igf2 allele is expressed in a mouse only if it is inherited from the mouse’s father—
If the DNA sequence of the gene does not correlate with activity, what does? The answer is that during the development of gametes, methyl groups are added to the DNA in the regulatory regions of imprinted genes in one sex only. We saw earlier that DNA of genes that are shut down for an entire lifetime are usually highly methylated. However, it is important to note that DNA methylation is one of several epigenetic marks associated with the long-
461
Let’s turn again to the mouse Igf2 and H19 genes to see how imprinting works at the molecular level. These two genes are located in a cluster of imprinted genes on mouse chromosome 7. There are an estimated 100 imprinted genes in the mouse, and most are found in clusters comprising from 3 to 11 imprinted genes. (Humans have most of the same clustered imprinted genes as those in the mouse.) In all cases examined, there is a specific pattern of DNA methylation for each gene copy of an imprinted gene. For the Igf2–
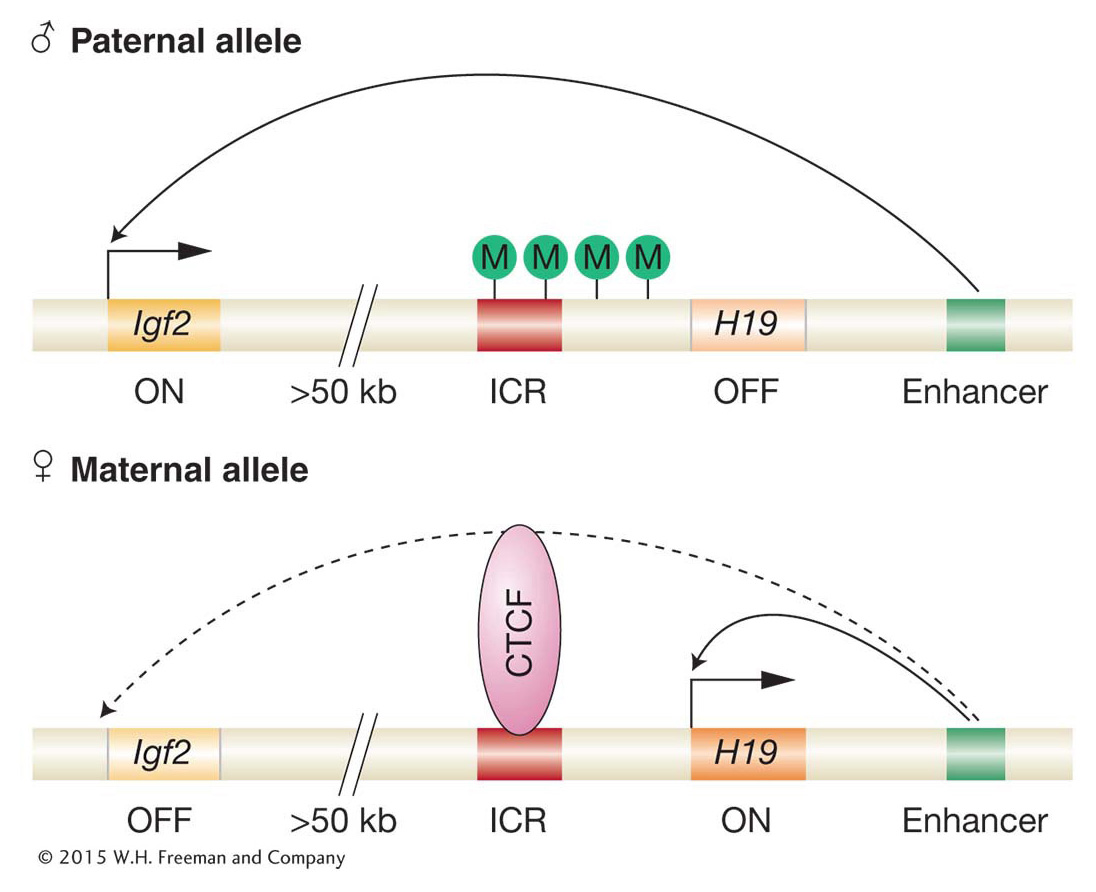
How does methylation control which of the two genes is active? Methylation acts as a block to the binding of proteins needed for transcription. Only the unmethylated (female) ICR can bind a regulatory protein called CTCF. When bound, CTCF acts as an enhancer-
Thus, we see how an enhancer-
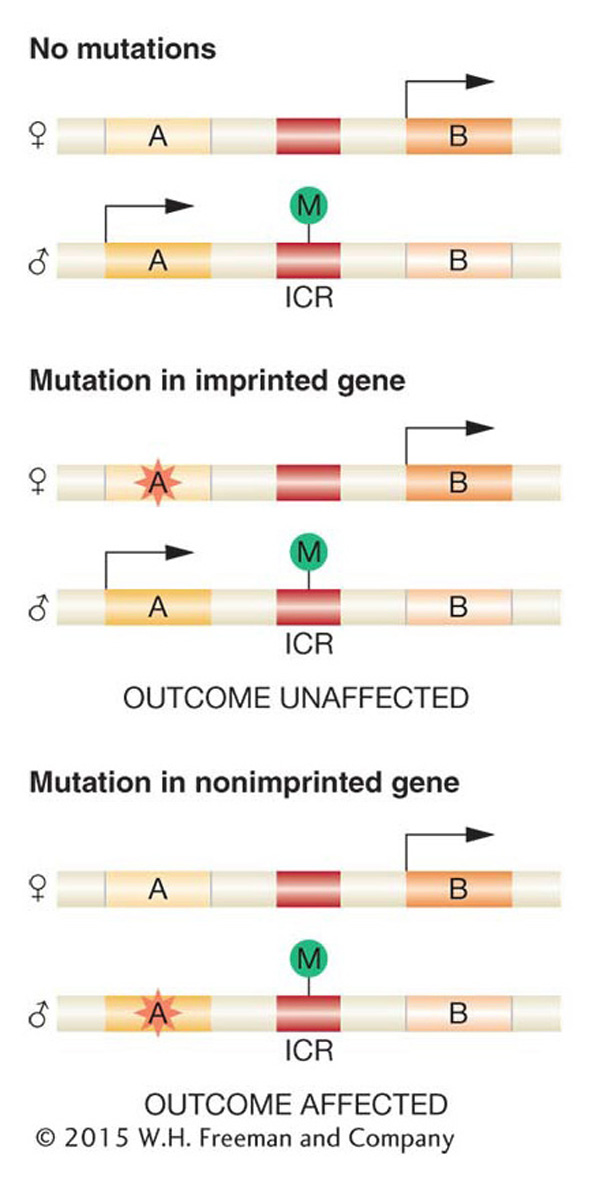
Note that parental imprinting can greatly affect pedigree analysis. Because the inherited allele from one parent is inactive, a mutation in the allele inherited from the other parent will appear to be dominant, whereas, in fact, the allele is expressed because only one of the two homologs is active for this gene. Figure 12-28 shows how a mutation in an imprinted gene can have different outcomes on the phenotype of the organism if inherited from the male or from the female parent.
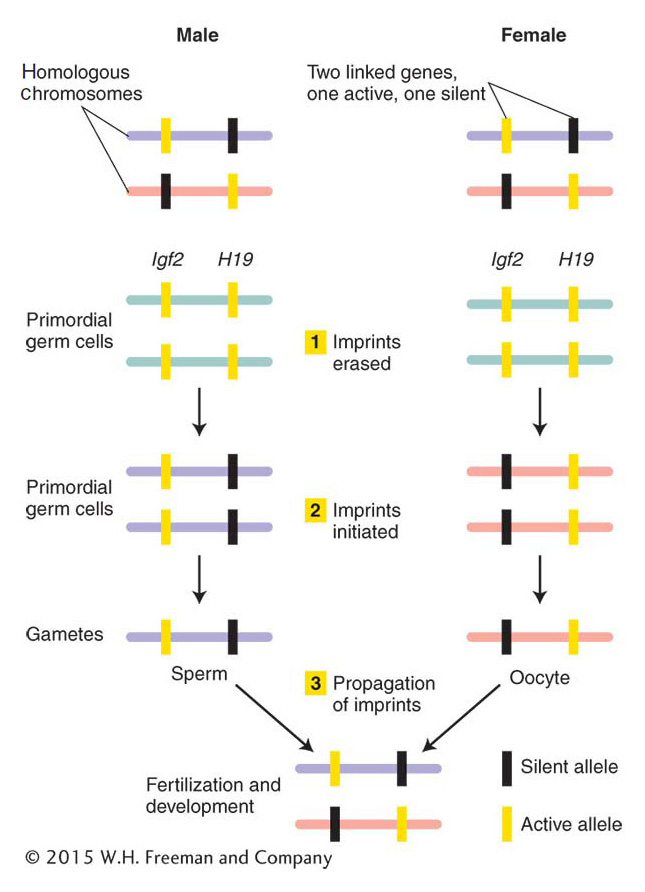
Many steps are required for imprinting (Figure 12-29). Soon after fertilization, mammals set aside cells that will become their germ cells. Imprints are erased before the germ cells form. Without their distinguishing mark of DNA methylation, these genes are now said to be epigenetically equivalent. As these primordial germ cells become fully formed gametes, imprinted genes receive the sex-
But what about Dolly and other cloned mammals?
Many thought that genomic imprinting would lead to a requirement that both male and female germ cells participate in mammalian embryo development. That is, male and female gametes contain different subsets of imprinted genes; so germ cells of both sexes must participate for the embryo to have a full complement of active imprinted genes. Why, then, are mammals such as Dolly and, more recently, cloned pigs, cats, dogs, and cows that were derived from somatic nuclei able to survive and even flourish? After all, as already noted, the mutation of even a single imprinted gene can be lethal or can lead to serious disease.
At this point, scientists do not understand why the cloning of many mammalian species has been successful. However, despite these successes, cloning is extremely inefficient in all species tested. For most experiments, a successful clone is an exceedingly rare event, requiring hundreds, even thousands of attempts. One could argue that the failure of most cloned embryos to develop into viable organisms is a testament to the importance of the epigenetic mechanisms of gene regulation in eukaryotes. As such, it illustrates how knowledge of the complete DNA sequence of all genes in an organism is only a first step in understanding how eukaryotic genes are regulated.
462
Silencing an entire chromosome: X-chromosome inactivation
The epigenetic phenomenon called X-
There is an exception to this generalization, however. All individuals would not produce the same number of transcripts of genes located on the sex chromosomes if both X chromosomes were expressed in females. As discussed in Chapter 2, the number of the X and Y sex chromosomes differs between the sexes, with female mammals having two X chromosomes and males having only one. The mammalian X chromosome is thought to contain about 1000 genes. Females have twice as many copies of these X-
463
This dosage imbalance is corrected by a process called dosage compensation, which makes the amount of most gene products from the two copies of the X chromosome in females equivalent to the single dose of the X chromosome in males. In mammals, dosages are made equivalent by randomly inactivating one of the two X chromosomes in each cell at an early stage in development. This inactive state is then propagated to all progeny cells. (In the germ line, the second X chromosome becomes reactivated in oogenesis.) The inactivated chromosome, called a Barr body, can be seen in the nucleus as a darkly staining, highly condensed, heterochromatic structure.
X-
The mechanism that converts a fully functional X chromosome into heterochromatin is the subject of current investigations. The process is well characterized in the mouse, and X-
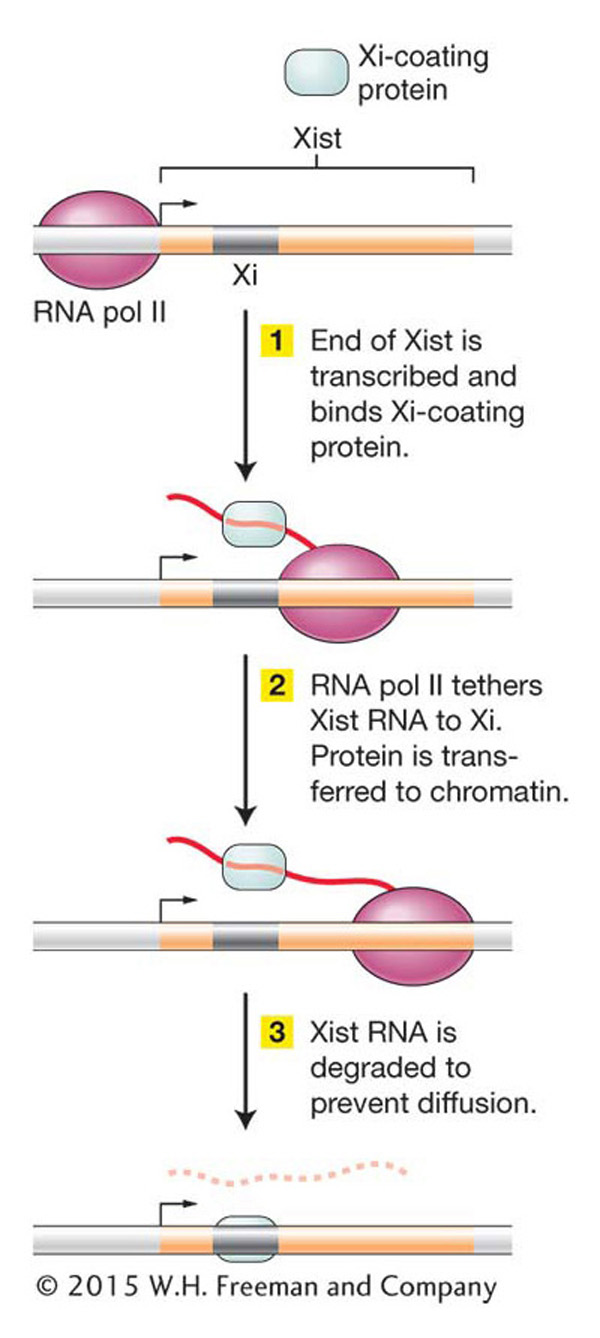
One interesting model for how transcription of an ncRNA could influence chromatin structure in Xi is shown in Figure 12-30. According to this model, as an ncRNA is transcribed by RNA pol II, proteins bind specifically to its sequences and catalyze the histone modifications that initiate heterochromatin formation. In this way, ncRNAs act as tethers to recruit chromatin-