13.1 The Genetic Approach to Development
For many decades, the study of embryonic development largely entailed the physical manipulation of embryos, cells, and tissues. Several key concepts were established about the properties of developing embryos through experiments in which one part of an embryo was transplanted into another part of the embryo. For example, the transplantation of a part of a developing amphibian embryo to another site in a recipient embryo was shown to induce the surrounding tissue to form a second complete body axis (Figure 13-2a). Similarly, transplantation of the posterior part of a developing chick limb bud to the anterior could induce extra digits, but with reversed polarity with respect to the normal digits (Figure 13-2b). These transplanted regions of the amphibian embryo and chick limb bud were termed organizers because of their remarkable ability to organize the development of surrounding tissues. The cells in the organizers were postulated to produce morphogens, molecules that induced various responses in surrounding tissue in a concentration-
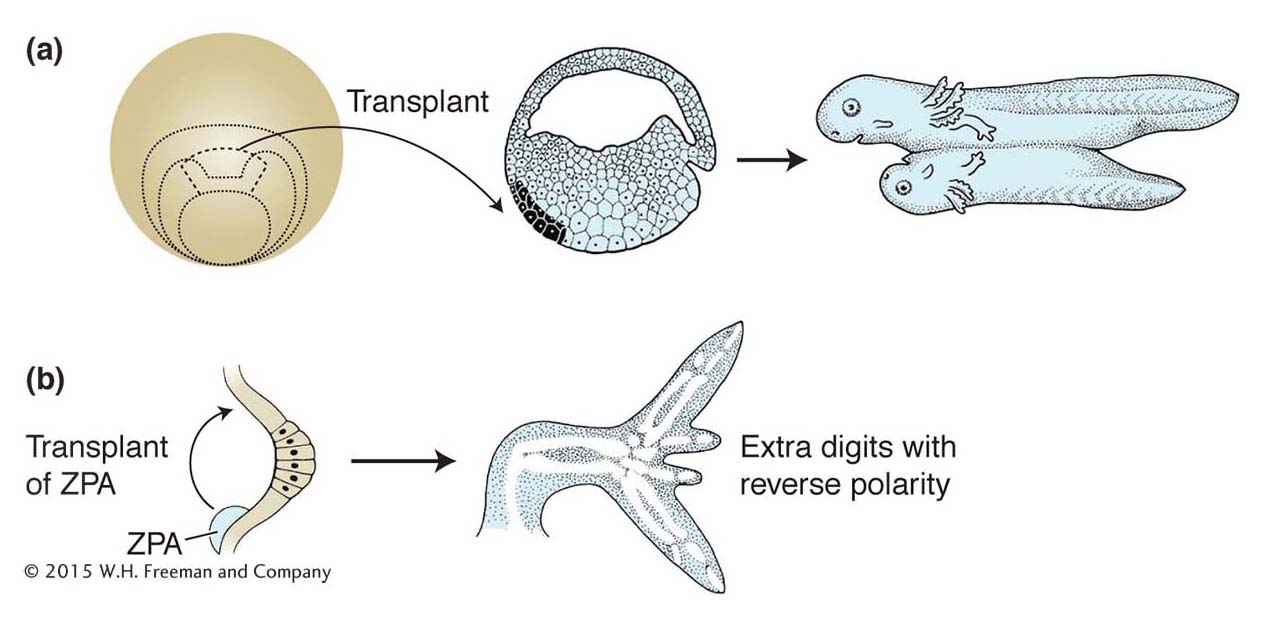
Although these experimental results were spectacular and fascinating, further progress in understanding the nature of organizers and morphogens stalled after their discovery in the first half of the 1900s. It was essentially impossible to isolate the molecules responsible for these activities by using biochemical separation techniques. Embryonic cells make thousands of substances—
The long impasse in defining embryology in molecular terms was broken by genetic approaches—
472
Drosophila
Mutational Analysis of Early Drosophila Development
The initial insights into the genetic control of pattern formation emerged from studies of the fruit fly Drosophila melanogaster. Drosophila development has proved to be a gold mine to researchers because developmental problems can be approached by the use of genetic and molecular techniques simultaneously.
The Drosophila embryo has been especially important in understanding the formation of the basic animal body plan. One important reason is that an abnormality in the body plan of a mutant is easily identified in the larval exoskeleton in the Drosophila embryo. The larval exoskeleton is a noncellular structure, made of a polysaccharide polymer called chitin that is produced as a secretion of the epidermal cells of the embryo. Each structure of the exoskeleton is formed from epidermal cells or cells immediately underlying that structure. With its intricate pattern of hairs, indentations, and other structures, the exoskeleton provides numerous landmarks to serve as indicators of the fates assigned to the many epidermal cells. In particular, there are many distinct anatomical structures along the anteroposterior (A–
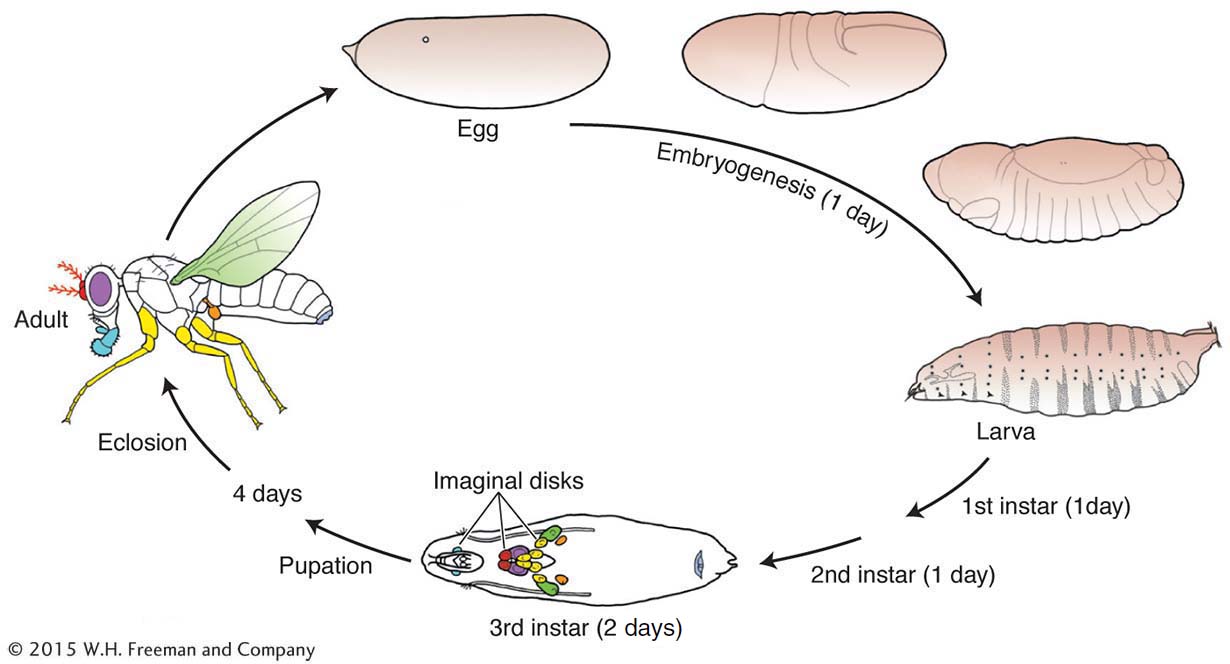
The development of the Drosophila adult body pattern takes a little more than a week (see diagram). Small populations of cells set aside during embryogenesis proliferate during three larval stages (instars) and differentiate in the pupal stage into adult structures. These set-
Genes that contribute to the Drosophila body plan can be cloned and characterized at the molecular level with ease. The analysis of the cloned genes often provides valuable information on the function of the protein product—
Using Knowledge from One Model Organism to Fast-
With the discovery that there are numerous homeobox genes within the Drosophila genome, similarities among the DNA sequences of these genes could be exploited in treasure hunts for other members of the homeotic-
From the genetic viewpoint, there are four key questions concerning the number, identity, and function of genes taking part in development:
Which genes are important in development?
Where in the developing animal and at what times are these genes active?
How is the expression of developmental genes regulated?
Through what molecular mechanisms do gene products affect development?
To address these questions, strategies had to be devised to identify, catalog, and analyze genes that control development. One of the first considerations in the genetic analysis of animal development was which animal to study. Of the millions of living species, which offered the most promise? The fruit fly Drosophila melanogaster emerged as the leading genetic model of animal development because its ease of rearing, rapid life cycle, cytogenetics, and decades of classical genetic analysis (including the isolation of many very dramatic mutants) provided important experimental advantages (see the Model Organism box on Drosophila above). The nematode worm Caenorhabditis elegans also presented many attractive features, most particularly its simple construction and well-
473
Through systematic and targeted genetic analysis, as well as comparative genomic studies, much of the genetic toolkit for the development of the bodies, body parts, and cell types of several different animal species has been defined. We will first focus on the genetic toolkit of Drosophila melanogaster because its identification was a source of major insights into the genetic control of development; its discovery catalyzed the identification of the genetic toolkit of other animals, including humans.
474