13.2 The Genetic Toolkit for Drosophila Development
Animal genomes typically contain about 13,000 to 22,000 genes. Many of these genes encode proteins that function in essential processes in all cells of the body (for example, in cellular metabolism or the biosynthesis of macromolecules). Such genes are often referred to as housekeeping genes. Other genes encode proteins that carry out the specialized tasks of various organ systems, tissues, and cells of the body such as the globin proteins in oxygen transport or antibody proteins that mediate immunity. Here, we are interested in a different set of genes, those concerned with the building of organs and tissues and the specification of cell types—
Toolkit genes of the fruit fly have generally been identified through the monstrosities or catastrophes that arise when they are mutated. Toolkit-
KEY CONCEPT
The genetic toolkit for animal development is composed of a small fraction of all genes. Only a small subset of the entire complement of genes in the genome affect development in discrete ways.Classification of genes by developmental function
One of the first tasks following the execution of a genetic screen for mutations is to sort out those of interest. Many mutations are lethal when hemi-
We will begin our inventory of the Drosophila toolkit by examining the genes that control the identity of segments and appendages. We do so for both historical and conceptual purposes. The genes controlling segmental and appendage identity were among the very first toolkit genes identified. Subsequent discoveries about their nature were sources of profound insights into not just how their products work, but also the content and workings of the toolkits of most animals. Furthermore, their spectacular mutant phenotypes indicate that they are among the most globally acting genes that affect animal form. Learning about these genes should whet our appetites for learning more about the whole toolkit that controls the development of animal form.
Homeotic genes and segmental identity
Among the most fascinating abnormalities to be described in animals are those in which one normal body part is replaced by another. Such homeotic transformations have been observed in many species in nature, including sawflies in which a leg forms in place of an antenna and frogs in which a thoracic vertebra forms in place of a cervical vertebra (Figure 13-3). Whereas only one member of a bilateral pair of structures is commonly altered in many naturally occurring variants, both members of a bilateral pair of structures are altered in homeotic mutants of fruit flies. In the former case, the alteration is not heritable, but homeotic mutants breed true from generation to generation.
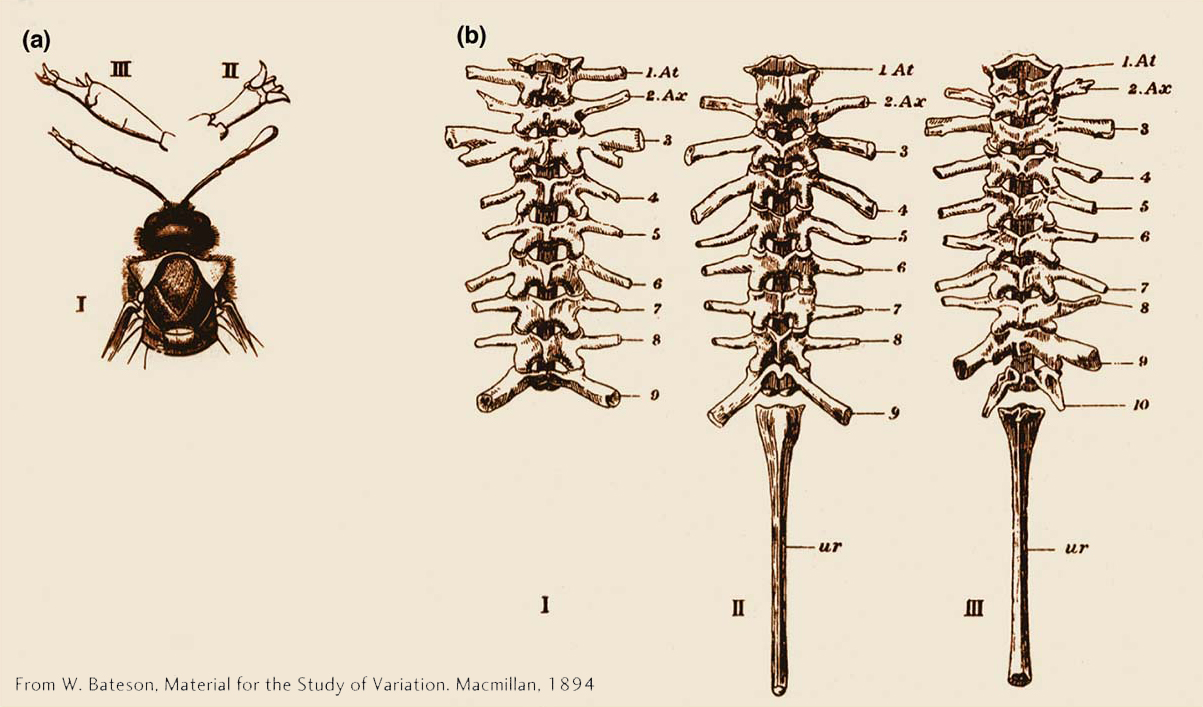
475
The scientific fascination with homeotic mutants stems from three properties. First, it is amazing that a single gene mutation can alter a developmental pathway so dramatically. Second, it is striking that the structure formed in the mutant is a well-
A mutation may cause a loss of homeotic gene function where the gene normally acts or it may cause a gain of homeotic function where the homeotic gene does not normally act. For example, the Ultrabithorax (Ubx) gene acts in the developing hind wing to promote hind-
476
Although homeotic genes were first identified through spontaneous mutations affecting adult flies, they are required throughout most of a fly’s development. Systematic searches for homeotic genes have led to the identification of eight loci, now referred to as Hox genes, that affect the identity of segments and their associated appendages in Drosophila. Generally, the complete loss of any Hox-gene function is lethal in early development. The dominant mutations that transform adults are viable in heterozygotes because the wild-
Organization and expression of Hox genes
A most intriguing feature of Hox genes is that they are clustered together in two gene complexes that are located on the third chromosome of Drosophila. The Bithorax complex contains three Hox genes, and the Antennapedia complex contains five Hox genes. Moreover, the order of the genes in the complexes and on the chromosome corresponds to the order of body regions, from head to tail, that are influenced by each Hox gene (Figure 13-4).
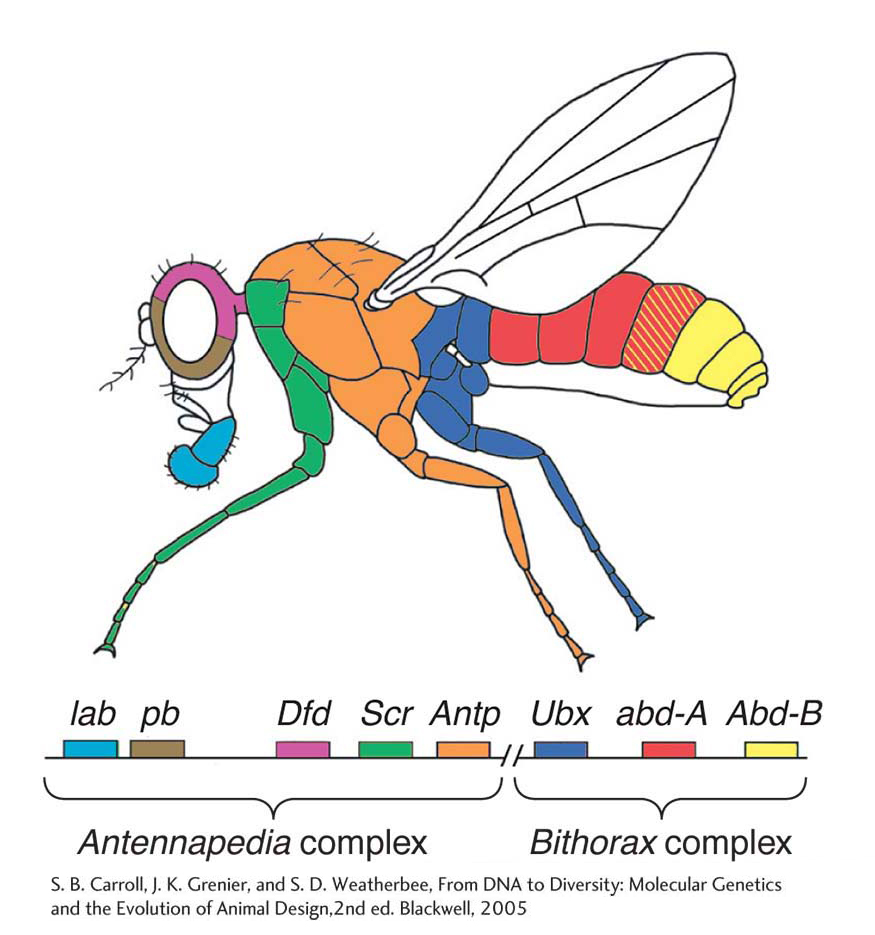
The relation between the structure of the Hox-gene complexes and the phenotypes of Hox-gene mutants was illuminated by the molecular characterization of the genes. Molecular cloning of the sequences encompassing each Hox locus provided the means to analyze where in the developing animal each gene is expressed. These spatial aspects of gene expression and gene regulation are crucial to understanding the logic of the genetic control of development. In regard to the Hox genes and other toolkit genes, the development of technology that made possible the visualization of gene and protein expression was crucial to understanding the relation among gene organization, gene function, and mutant phenotypes.
Two principal technologies for the visualization of gene expression in embryos or other tissues are (1) the expression of RNA transcripts visualized by in situ hybridization and (2) the expression of proteins visualized by immunological methods. Each technology depends on the isolation of cDNA clones representing the mature mRNA transcript and protein (Figure 13-5).
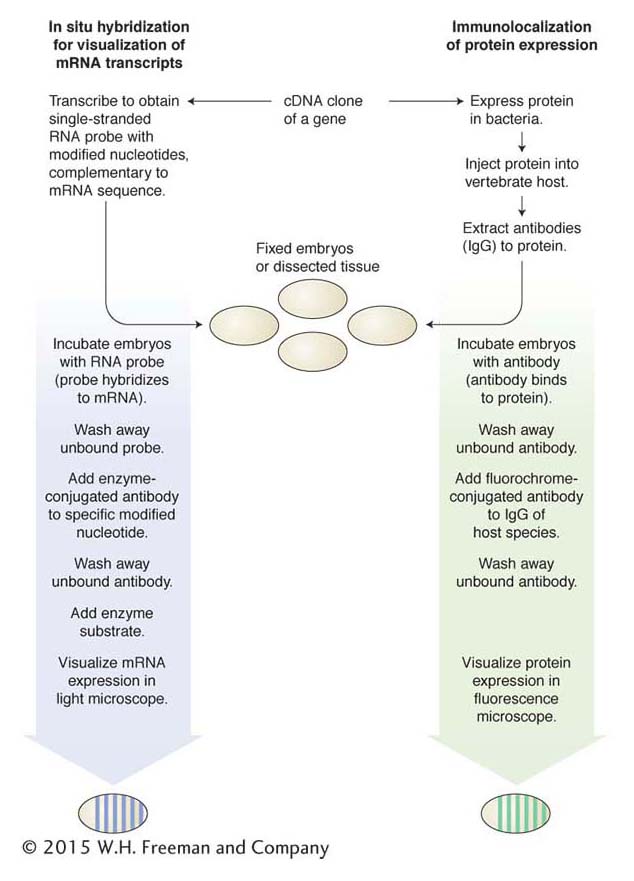
477
In the developing embryo, the Hox genes are expressed in spatially restricted, sometimes overlapping domains within the embryo (Figure 13-6). The genes are also expressed in the larval and pupal tissues that will give rise to the adult body parts.
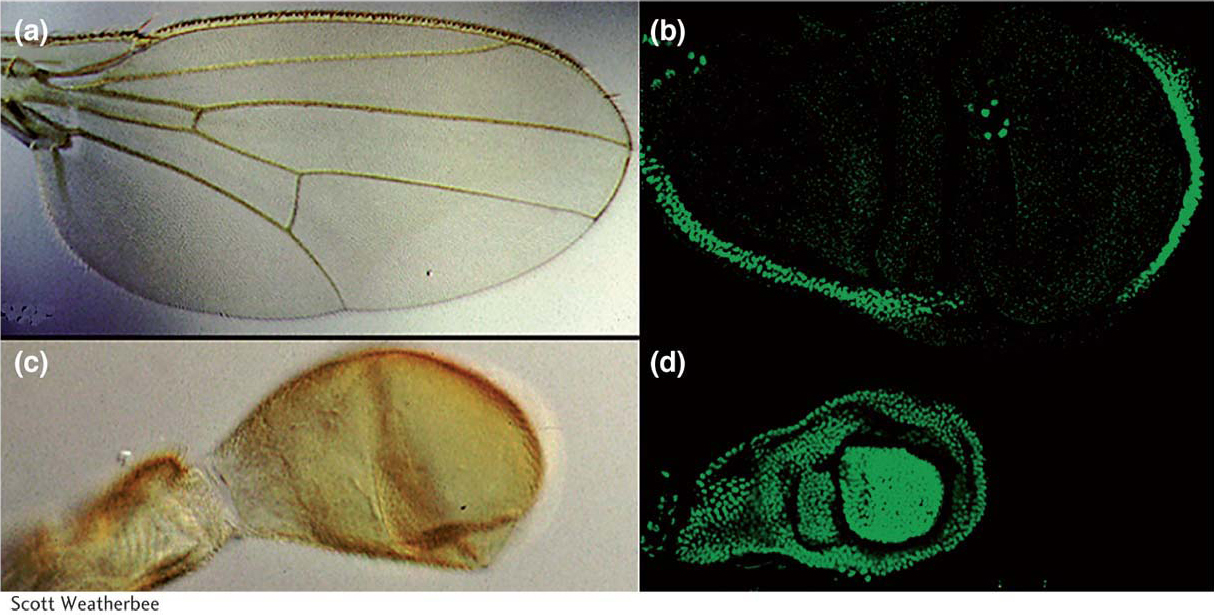
The patterns of Hox-gene expression (and other toolkit genes) generally correlate with the regions of the animal affected by gene mutations. For example, the dark blue shading in Figure 13-6 indicates where the Ubx gene is expressed. This Hox gene is expressed in the posterior thoracic and most of the abdominal segments of the embryo. The development of these segments is altered in Ubx mutants. Ubx is also expressed in the developing hind wing but not in the developing forewing (Figure 13-7), as one would expect knowing that Ubx promotes hind-
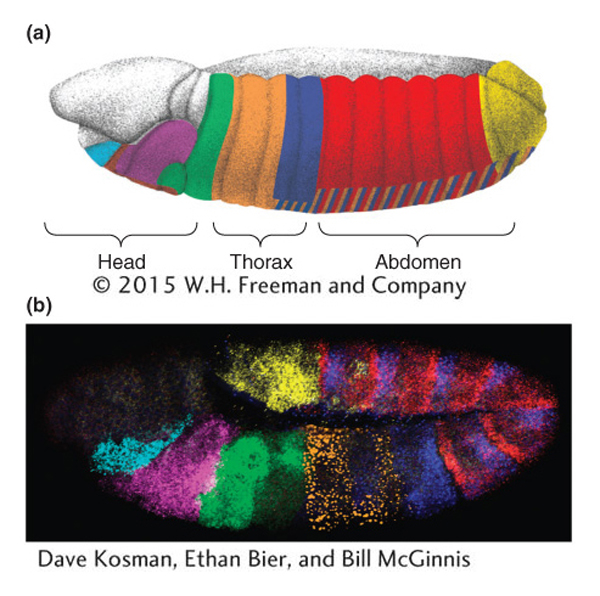
478
KEY CONCEPT
The spatial expression of toolkit genes is usually closely correlated with the regions of the animal affected by gene mutations.It is crucial to distinguish the role of Hox genes in determining the identity of a structure from that governing its formation. In the absence of function of all Hox genes, segments form, but they all have the same identity; limbs also can form, but they have antennal identity; and, similarly, wings can form, but they have forewing identity. Other genes control the formation of segments, limbs, and wings and will be described later. First, we must understand how Hox genes exert their dramatic effects on fly development.
The homeobox
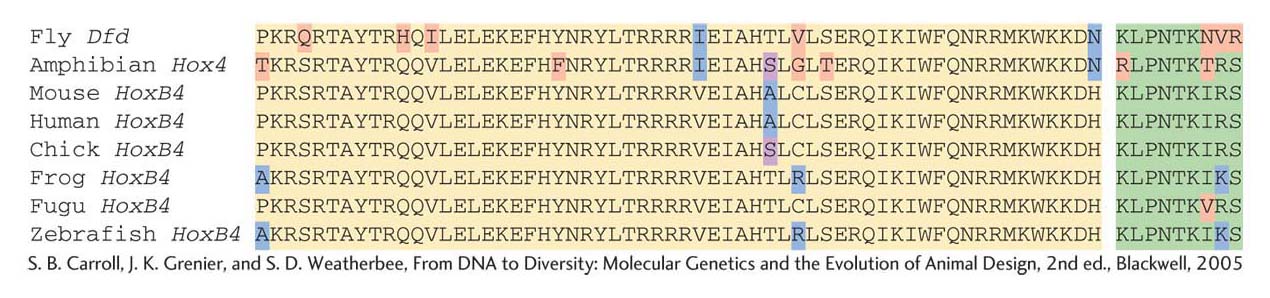
Because Hox genes have large effects on the identities of entire segments and other body structures, the nature and function of the proteins that they encode are of special interest. Edward Lewis, a pioneer in the study of homeotic genes, noted early on that the clustering of Bithorax complex genes suggested that the multiple loci had arisen by tandem duplication of an ancestral gene. This idea led researchers to search for similarities in the DNA sequences of Hox genes. They found that all eight Hox genes of the two complexes were similar enough to hybridize to each other. This hybridization was found to be due to a short region of sequence in each gene, 180 bp in length. This stretch of DNA sequence similarity, because of its presence in homeotic genes, was dubbed the homeobox. The homeobox encodes a protein domain, the homeodomain, containing 60 amino acids. The amino acid sequence of the homeodomain is very similar among the Hox proteins (Figure 13-8).

479
Although the discovery of a common protein motif in each of the Hox proteins was very exciting, further analysis of the structure of the homeodomain revealed that it forms a helix-
KEY CONCEPT
Many toolkit genes encode transcription factors that regulate the expression of other genes.We will examine how Hox proteins and other toolkit proteins orchestrate gene expression in development a little later. First, there is one more huge discovery to describe, which revealed that what we learn from fly Hox genes has very general implications for the animal kingdom.
Clusters of Hox genes control development in most animals
When the homeobox was discovered in fly Hox genes, it raised the question whether this feature was some peculiarity of these bizarre fly genes or was more widely distributed, in other insects or segmented animals, for example. To address this possibility, researchers searched for homeoboxes in the genomes of other insects, as well as earthworms, frogs, cows, and even humans. They found many homeoboxes in each of these animal genomes.
The similarities in the homeobox sequences from different species were astounding. Over the 60 amino acids of the homeodomain, some mouse and frog Hox proteins were identical with the fly sequences at as many as 59 of the 60 positions (Figure 13-9). In light of the vast evolutionary distances between these animals, more than 500 million years since their last common ancestor, the extent of sequence similarity indicates very strong pressure to maintain the sequence of the homeodomain.
480
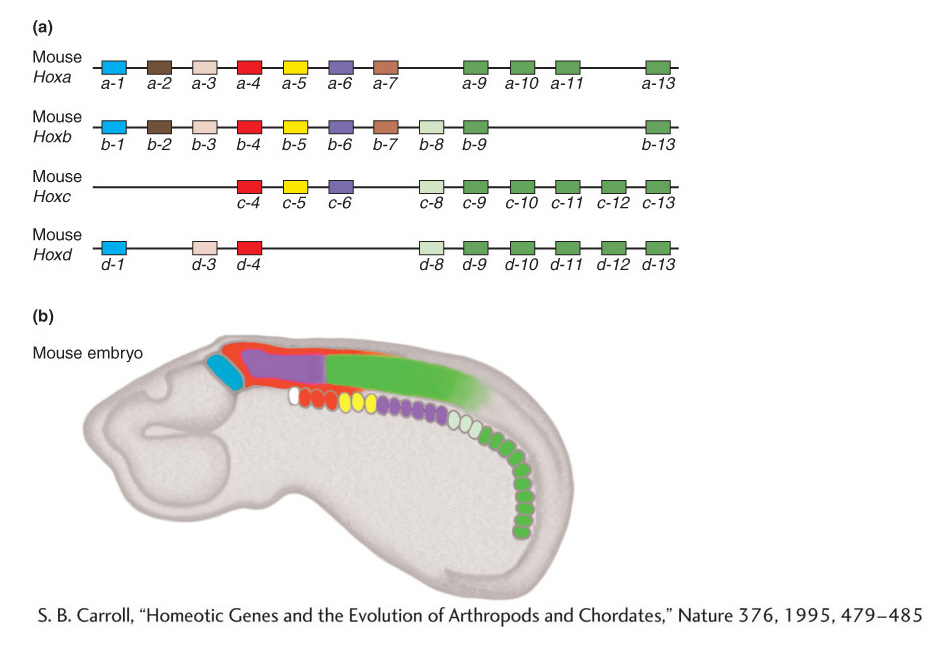
The existence of Hox genes with homeoboxes throughout the animal kingdom was entirely unexpected. Why different types of animals would possess the same regulatory genes was not obvious, which is why biologists were further surprised by the results when the organization and expression of Hox genes was examined in other animals. In vertebrates, such as the laboratory mouse, the Hox genes also are clustered together in four large gene complexes on four different chromosomes. Each cluster contains from 9 to 11 Hox genes, a total of 39 Hox genes altogether. Furthermore, the order of the genes in the mouse Hox complexes parallels the order of their most related counterparts in the fly Hox complexes, as well as in each of the other mouse Hox clusters (Figure 13-10a). This correspondence indicates that the Hox complexes of insects and vertebrates are related and that some form of Hox complex existed in their distant common ancestor. The four Hox complexes in the mouse arose by duplications of entire Hox complexes (perhaps of entire chromosomes) in vertebrate ancestors.
481
Why would such different animals have these sets of genes in common? Their deep, common ancestry indicates that Hox genes play some fundamental role in the development of most animals. That role is apparent from analyses of how the Hox genes are expressed in different animals. In vertebrate embryos, adjacent Hox genes also are expressed in adjacent or partly overlapping domains along the anteroposterior body axis. Furthermore, the order of the Hox genes in the complexes corresponds to the head-
The Hox-gene expression patterns of vertebrates suggested that they also specify the identity of body regions, and subsequent analyses of Hox-
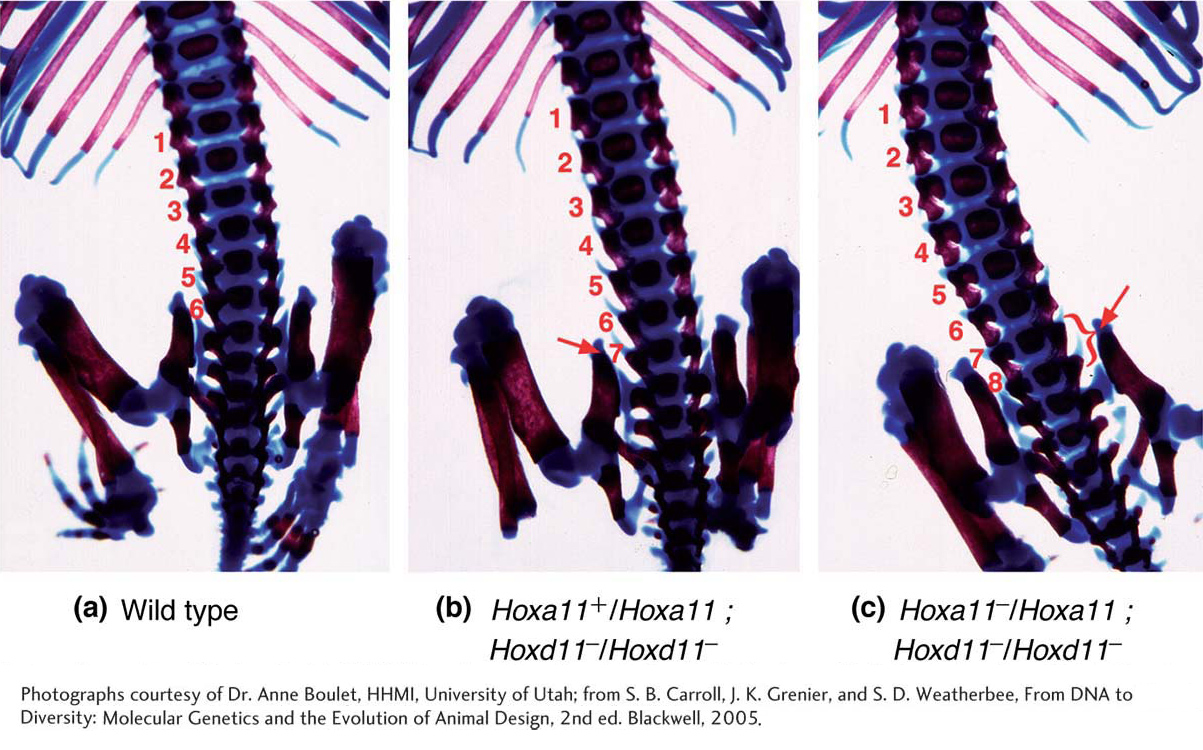
482
KEY CONCEPT
Despite great differences in anatomy, many toolkit genes are common to a broad array of different animal phyla.Now let’s take an inventory of the rest of the toolkit to see what other general principles emerge.