13.4 Spatial Regulation of Gene Expression in Development
We have seen that toolkit genes are expressed in reference to coordinates in the embryo. But how are the spatial coordinates of the developing embryo conveyed as instructions to genes, to turn them on and off in precise patterns? As described in Chapters 11 and 12, the physiological control of gene expression in bacteria and simple eukaryotes is ultimately governed by sequence-specific DNA-binding proteins acting on cis-acting regulatory elements (for example, operators and upstream-activation-sequence, or UAS, elements). Similarly, the spatial control of gene expression during development is largely governed by the interaction of transcription factors with cis-acting regulatory elements. However, the spatial and temporal control of gene regulation in the development of a three-dimensional multicellular embryo requires the action of more transcription factors on more numerous and more complex cis-acting regulatory elements.
To define a position in an embryo, regulatory information must exist that distinguishes that position from adjacent regions. If we picture a three-dimensional embryo as a globe, then positional information must be specified that indicates longitude (location along the anteroposterior axis), latitude (location along the dorsoventral axis), and altitude or depth (position in the germ layers). We will illustrate the general principles of how the positions of gene expression are specified with three examples. These examples should be thought of as just a few snapshots of the vast number of regulatory interactions that govern fly and animal development. Development is a continuum in which every pattern of gene activity has a preceding causal basis. The entire process includes tens of thousands of regulatory interactions and outputs.
We will focus on a few connections between genes in different levels of the hierarchies that lay out the basic segmental body plan and on nodal points where key genes integrate multiple regulatory inputs and respond by producing simpler gene-expression outputs.
Maternal gradients and gene activation
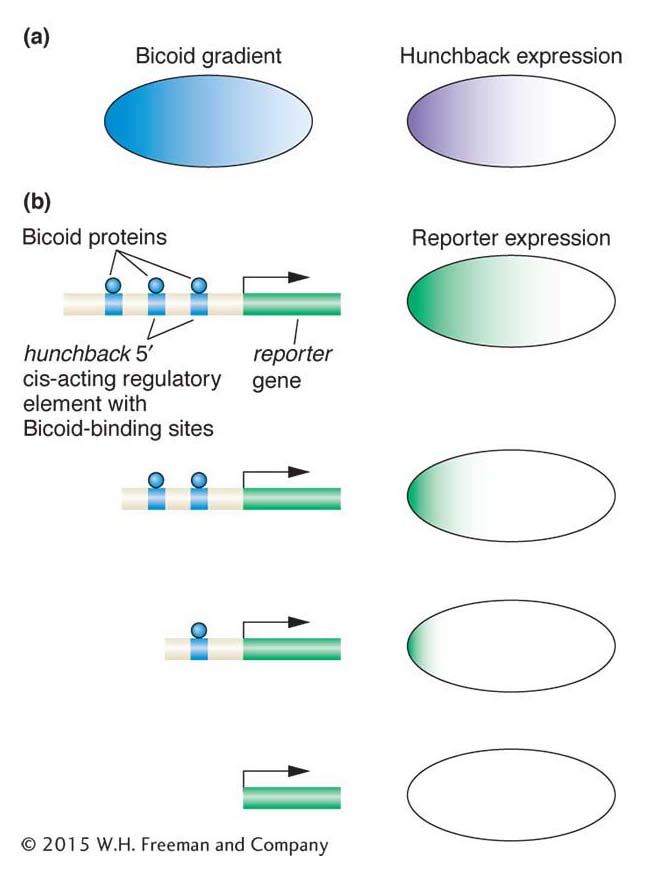
Figure 13-18: Gap genes are activated by specific maternally provided proteins
Figure 13-18: The Bicoid protein activates zygotic expression of the hunchback gene. (a) Bicoid protein expression is graded along the anteroposterior axis. The hunchback gap gene is expressed in the anterior half of the zygote. (b) The Bicoid protein (blue) binds to three sites 5′ of the hunchback gene. When this 5′ DNA is placed upstream of a reporter gene, reportergene expression recapitulates the pattern of hunchback expression (top right). However, progressive deletion of one, two, or all three Bicoid-binding sites either leads to more restricted expression of the reporter gene or abolishes it altogether. These observations show that the level and pattern of hunchback expression are controlled by Bicoid through its binding to hunchback DNA regulatory sequences.
The Bicoid protein is a homeodomain-type transcription factor that is translated from maternally derived mRNA that is deposited in the egg and localized at the anterior pole. Because the early Drosophila embryo is a syncytium with all nuclei in one cytoplasm, and lacks any cell membranes that would impede the diffusion of protein molecules, the Bicoid protein can diffuse through the cytoplasm. This diffusion establishes a protein concentration gradient (Figure 13-18a): the Bicoid protein is highly concentrated at the anterior end, and this concentration gradually decreases as distance from that end increases, until there is very little Bicoid protein beyond the middle of the embryo. This concentration gradient provides positional information about the location along the anteroposterior axis. A high concentration means anterior end, a lower concentration means middle, and so on. Thus, a way to ensure that a gene is activated in only one location along the axis is to link gene expression to the concentration level. A case in point is the gap genes, which must be activated in specific regions along the axis.
Several zygotic genes, including gap genes, are regulated by different levels of the Bicoid protein. For example, the hunchback gene is a gap gene activated in the zygote in the anterior half of the embryo. This activation is through direct binding of the Bicoid protein to three sites 5′ of the promoter of the hunchback gene. Bicoid binds to these sites cooperatively; that is, the binding of one Bicoid protein molecule to one site facilitates the binding of other Bicoid molecules to nearby sites.
In vivo experiments can demonstrate that the activation of hunchback depends on the concentration gradient. These tests require linking gene regulatory sequences to a reporter gene (an enzyme-encoding gene such as the LacZ gene or the green fluorescent protein of jellyfish), introducing the DNA construct into the fly germ line, and monitoring reporter expression in the embryo offspring of transgenic flies (a general overview of the method is shown in Figure 13-19). The wild-type sequences 5′ of the hunchback gene are sufficient to drive reporter expression in the anterior half of the embryo. Importantly, deletions of Bicoid-binding sites in this cis-acting regulatory element reduce or abolish reporter expression (see Figure 13-18b). More than one Bicoid site must be occupied to generate a sharp boundary of reporter expression, which indicates that a threshold concentration of Bicoid protein is required to occupy multiple sites before gene expression is activated. A gap gene with fewer binding sites will not be activated at locations with lower concentration of Bicoid protein.
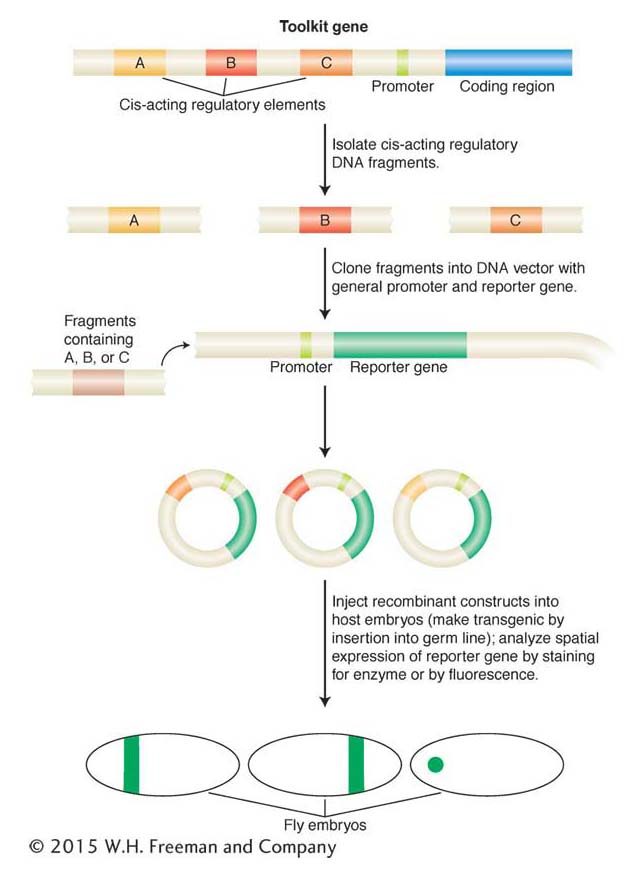
Figure 13-19: Analysis of cis-acting regulatory elements with reporter genes
Figure 13-19: Toolkit loci (such as hunchback, as described in the text) often contain multiple independent cis-acting regulatory elements that control gene expression in different places or at different times during development or both (for example, A, B, C, here). These elements are identified by their ability, when placed in cis to a reporter gene and inserted back into a host genome, to control the pattern, timing, or level, or all three, of reportergene expression. In this example, each element drives a different pattern of gene expression in a fly embryo. Most reporter genes encode enzymes or fluorescent proteins that can be easily visualized.
Each gap gene contains cis-acting regulatory elements with different arrangements of binding sites, and these binding sites may have different affinities for the Bicoid protein. Consequently, each gap gene is expressed in a unique distinct domain in the embryo, in response to different levels of Bicoid and other transcription-factor gradients. A similar theme is found in the patterning of the dorsoventral axis: cis-acting regulatory elements contain different numbers and arrangements of binding sites for Dorsal and other dorsoventral transcription factors. Consequently, genes are activated in discrete domains along the dorsoventral axis.
KEY CONCEPT
The concentration-dependent response of genes to graded inputs is a crucial feature of gene regulation in the early Drosophila embryo. The cis-acting regulatory elements governing distinct responses contain different numbers and arrangements of transcription-factor-binding sites.
Drawing stripes: Integration of gap-protein inputs
The expression of each pair-rule gene in seven stripes is the first sign of the periodic organization of the embryo and future animal. How are such periodic patterns generated from prior aperiodic information? Before the molecular analysis of pair-rule-gene regulation, several models were put forth to explain stripe formation. Every one of these ideas viewed all seven stripes as identical outputs in response to identical inputs. However, the actual way in which the patterns of a few key pair-rule genes are encoded and generated is one stripe at a time. The solution to the mystery of stripe generation highlights one of the most important concepts concerning the spatial control of gene regulation in developing animals; namely, the distinct cis-acting regulatory elements of individual genes are controlled independently.
The key discovery was that each of the seven stripes that make up the expression patterns of the even-skipped and hairy pair-rule genes is controlled independently. Consider the second stripe expressed by the even-skipped gene (Figure 13-20a). This stripe lies within the broad region of hunchback expression and on the edges of the regions of expression of two other gap proteins, Giant and Krüppel (Figure 13-20b). Thus, within the area of the future stripe, there will be large amounts of Hunchback protein and small amounts of Giant protein and Krüppel protein. There will also be a certain concentration of the maternal-effect Bicoid protein. No other stripe of the embryo will contain these proteins in these proportions. The formation of stripe 2 is controlled by a specific cis-acting regulatory element, an enhancer, that contains a number of binding sites for these four proteins (Figure 13-20c). Detailed analysis of the eve stripe 2 cis-acting regulatory element revealed that the position of this “simple” stripe is controlled by the binding of these four aperiodically distributed transcription factors, including one maternal protein and three gap proteins.
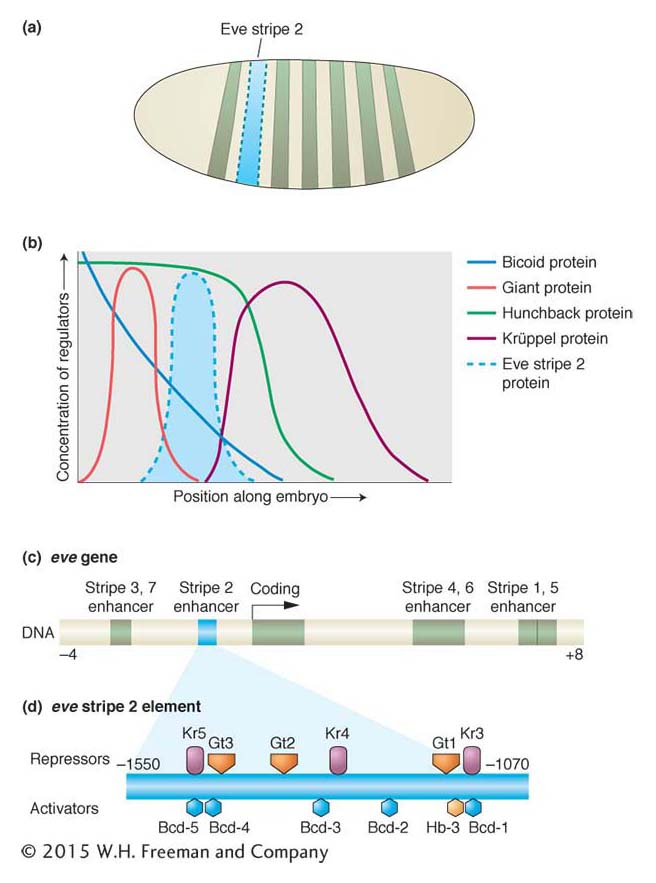
Figure 13-20: Combinations of maternal-effect and gap proteins control individual pair-rule stripe formation
Figure 13-20: Regulation of a pair-rule stripe: combinatorial control of an independent cis-acting regulatory element. (a) The regulation of the eve stripe 2 cis-acting regulatory element controls the formation of the second stripe of eve expression in the early embryo, just one of seven stripes of eve expression. (b) The stripe forms within the domains of the Bicoid and Hunchback proteins and at the edge of the Giant and Krüppel gap proteins. Bcd and Hb are activators, Gt and Kr are repressors of the stripe. (c) The eve stripe 2 element is just one of several cis-acting regulatory elements of the eve gene, each of which controls different parts of eve expression. The eve stripe 2 element spans from about 1 to 1.7 kb upstream of the eve transcription unit. (d) Within the eve stripe 2 element, several binding sites exist for each transcription factor (repressors are shown above the element, activators below). The net output of this combination of activators and repressors is expression of the narrow eve stripe.
Specifically, the eve stripe 2 element contains multiple sites for the maternal Bicoid protein and the Hunchback, Giant, and Krüppel gap proteins (Figure 13-20d). Mutational analyses of different combinations of binding sites revealed that Bicoid and Hunchback activate the expression of the eve stripe 2 element over a broad region. The Giant and Krüppel proteins are repressors that sharpen the boundaries of the stripe to just a few cells wide. The eve stripe 2 element acts, then, as a genetic switch, integrating multiple regulatory protein activities to produce one stripe from three to four cells wide in the embryo.
The entire seven-striped periodic pattern of even-skipped expression is the sum of different sets of inputs into separate cis-acting regulatory elements. The enhancers for other stripes contain different combinations of protein binding sites.
KEY CONCEPT
The regulation of cis-acting regulatory elements by combinations of activators and repressors is a common theme in the spatial regulation of gene expression. Complex patterns of inputs are often integrated to produce simpler patterns of outputs.
Making segments different: integration of Hox inputs
The combined and sequential activity of the maternal-effect, gap, pair-rule, and segment-polarity proteins establishes the basic segmented body plan of the embryo and larva. How are the different segmental identities established by Hox proteins? This process has two aspects. First, the Hox genes are expressed in different domains along the anteroposterior axis. Hox-gene expression is largely controlled by segmentation proteins, especially gap proteins, through mechanisms that are similar to those already described herein for hunchback and eve stripe 2 (as well as some cross-regulation by Hox proteins of other Hox genes). The regulation of Hox genes will not be considered in depth here. The second aspect of Hox control of segmental identity is the regulation of target genes by Hox proteins. We will examine one example that nicely illustrates how a major feature of the fruit fly’s body plan is controlled through the integration of many inputs by a single cis-acting regulatory element.
The paired limbs, mouthparts, and antennae of Drosophila each develop from initially small populations of about 20 cells in different segments. Different structures develop from the different segments of the head and thorax, whereas the abdomen is limbless. The first sign of the development of these structures is the activation of regulatory genes within small clusters of cells, which are called the appendage primordia. The expression of the Distal-less (Dll) gene marks the start of the development of the appendages. This gene is one of the key targets of the Hox genes, and its function is required for the subsequent development of the distal parts of each of these appendages. The small clusters of cells expressing Distal-less arise in several head segments and in each of the three thoracic segments but not in the abdomen (Figure 13-21a).
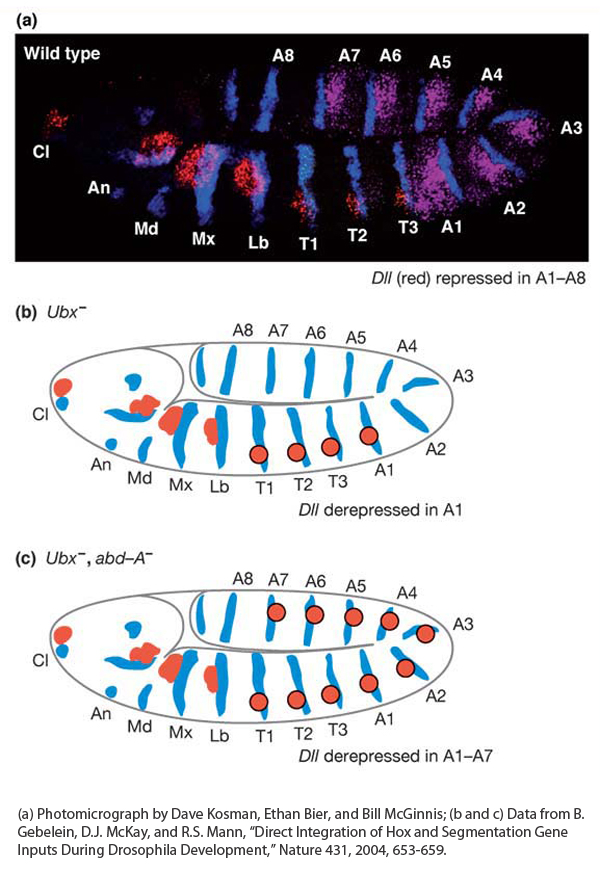
Figure 13-21: Hox proteins repress appendage formation in the abdomen
Figure 13-21: The absence of limbs in the abdomen is controlled by Hox genes. (a) The expression of the Distal-less (Dll) gene (red) marks the position of future appendages, expression of the Hox gene Ultrabithorax (purple) marks the position of the abdominal segments A1 through A7, and expression of the engrailed gene (blue) marks the posterior of each segment. (b) Schematic representation of Ubx− embryo showing that Dll expression (red circles) is derepressed in segment A1. (c) Schematic representation of Ubx−abd-A− embryo showing that Dll expression (red circles) is derepressed in the first seven abdominal segments.
[(a) Photomicrograph by Dave Kosman, Ethan Bier, and Bill McGinnis; (b and c) Data from B. Gebelein, D. J. McKay, and R. S. Mann, “Direct Integration of Hox and Segmentation Gene Inputs During Drosophila Development,” Nature 431, 2004, 653-659.]
How is Distal-less expression restricted to the more anterior segments? By repressing its expression in the abdomen. Several lines of evidence have revealed that the Distal-less gene is repressed by two Hox proteins—the Ultrabithorax and Abdominal-A proteins—working in collaboration with two segmentation proteins. Notice in Figure 13-6 that Ultrabithorax is expressed in abdominal segments one through seven, and Abdominal-A is expressed in abdominal segments two through seven, overlapping with all but the first segment covered by Ultrabithorax. In Ultrabithorax mutant embryos, Distal-less expression expands to the first abdominal segment (Figure 13-21b), and in Ultrabithorax/Abdominal-A double-mutant embryos, Distal-less expression extends through the first seven abdominal segments (Figure 13-21c), indicating that both proteins are required for the repression of Distal-less expression in the abdomen.
The cis-acting regulatory element responsible for Distal-less expression in the embryo has been identified and characterized in detail (Figure 13-22a). It contains two binding sites for the Hox proteins. If these two binding sites are mutated such that the Hox proteins cannot bind, Distal-less expression is derepressed in the abdomen (Figure 13-22b). Several additional proteins collaborate with the Hox proteins in repressing Distal-less. Two are proteins encoded by segment-polarity genes, Sloppy-paired (Slp) and engrailed (en). The Sloppy-paired and Engrailed proteins are expressed in stripes that mark the anterior and posterior compartments of each segment, respectively. Each protein also binds to the Distal-less cis-acting regulatory element. When the Sloppy-paired-binding site is mutated in the cis-acting regulatory element, reportergene expression is derepressed in the anterior compartments of abdominal segments (Figure 13-22c). When the Engrailed-binding site is mutated, reporter expression is derepressed in the posterior compartments of each abdominal segment (Figure 13-22d). And when the binding sites for both proteins are mutated, reportergene expression is derepressed in both compartments of each abdominal segment, just as when the Hox-binding sites are mutated (Figure 13-22e). Two other proteins, called Extradenticle and Homothorax, which are broadly expressed in every segment, also bind to the Distal-less cis-acting regulatory element and are required for transcriptional repression in the abdomen (Figure 13-22f).
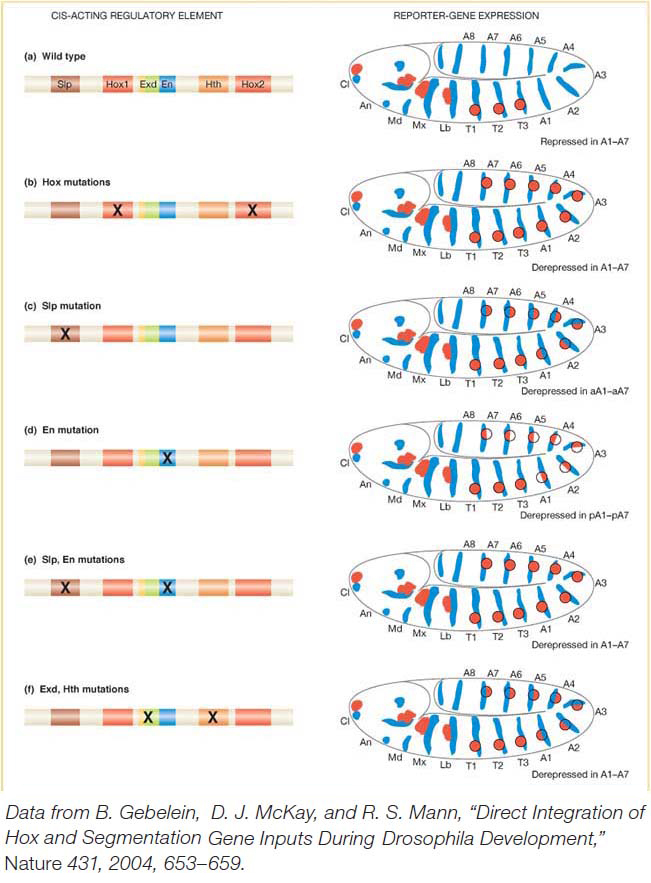
Figure 13-22: Hox proteins and segment-polarity proteins control appendage location
Figure 13-22: Integration of Hox and segmentation-protein inputs by a cis-acting regulatory element. (a) Left: A cis-acting regulatory element of the Dll gene governs the repression of D//expressioninthe abdomen by a set of transcription factors. (a) Right: Dll expression (red) extends to the thorax but not into the abdomen in a wild-type embryo. (b-f) Mutations in the respective binding sites shown derepress Dll expression in various patterns in the abdomen. Binding sites are: Slp, Sloppy-paired; Hox1 and Hox2, Ultrabithorax and Abdominal-A; Exd, Extradenticle; En, Engrailed; Hth, Homothorax.
[Data from B. Gebelein, D. J. McKay, and R. S. Mann, “Direct Integration of Hox and Segmentation Gene Inputs During Drosophila Development,” Nature 431, 2004, 653-659.]
Thus, altogether, two Hox proteins and four other transcription factors bind within a span of 57 base pairs and act together to repress Distal-less expression and, hence, appendage formation in the abdomen. The repression of Distal-less expression is a clear demonstration of how Hox proteins regulate segment identity and the number of reiterated body structures. It is also a good illustration of how diverse regulatory inputs act combinatorially on cis-acting regulatory elements. In this instance, the presence of Hox-binding sites is not sufficient for transcriptional repression: collaborative and cooperative interactions are required among several proteins to fully repress gene expression in the abdomen.
KEY CONCEPT
Combinatorial and cooperative regulation of gene transcription imposes greater specificity on spatial patterns of gene expression and allows for their greater diversity.Although evolutionary diversity has not been explicitly addressed in this chapter, the presence of multiple independent cis-acting regulatory elements for each toolkit gene has profound implications for the evolution of form. Specifically, the modularity of these elements allows for changes in one aspect of gene expression independent of other gene functions. The evolution of gene regulation plays a major role in the evolution of development and morphology. We will return to this topic in Chapter 20.




