13.5 Post-transcriptional Regulation of Gene Expression in Development
Although transcriptional regulation is a major means of restricting the expression of gene products to defined areas during development, it is not at all the exclusive means of doing so. Alternative RNA splicing also contributes to gene regulation, and so does the regulation of mRNA translation by proteins and microRNAs (miRNAs). In each case, regulatory sequences in RNA are recognized—by splicing factors, mRNA-binding proteins, or miRNAs—and govern the structure of the protein product, its amount, or the location where the protein is produced. We will look at one example of each type of regulatory interaction at the RNA level.
RNA splicing and sex determination in Drosophila
A fundamental developmental decision in sexually reproducing organisms is the specification of sex. In animals, the development of many tissues follows different paths, depending on the sex of the individual animal. In Drosophila, many genes have been identified that govern sex determination through the analysis of mutant phenotypes in which sexual identity is altered or ambiguous.
The doublesex (dsx) gene plays a central role in governing the sexual identity of somatic (non-germ-line) tissue. Null mutations in dsx cause females and males to develop as intermediate intersexes, which have lost the distinct differences between male and female tissues. Although dsx function is required in both sexes, different gene products are produced from the locus in different sexes. In males, the product is a specific, longer isoform, DsxM, that contains a unique C-terminal region of 150 amino acids not found in the female-specific isoform DsxF, which instead contains a unique 30 amino acid sequence at its carboxyl terminus. Each form of the Dsx protein is a DNA-binding transcription factor that apparently binds the same DNA sequences. However, the activities of the two isoforms differ: DsxF activates certain target genes in females that DsxM represses in males.
The alternative forms of the Dsx protein are generated by alternative splicing of the primary dsx RNA transcript. Thus, in this case, the choice of splice sites must be regulated to produce mature mRNAs that encode different proteins. The various genetic factors that influence Dsx expression and sex determination have been identified by mutations that affect the sexual phenotype.
One key regulator is the product of the transformer (tra) gene. Whereas null mutations in tra have no effect on males, XX female flies bearing tra mutations are transformed into the male phenotype. The Tra protein is an alternative splicing factor that affects the splice choices in the dsx RNA transcript. In the presence of Tra (and a related protein Tra2), a splice occurs that incorporates exon 4 of the dsx gene into the mature dsxF transcript (Figure 13-23), but not exons 5 and 6. Males lack the Tra protein; so this splice does not occur, and exons 5 and 6 are incorporated into the dsxM transcript, but not exon 4.
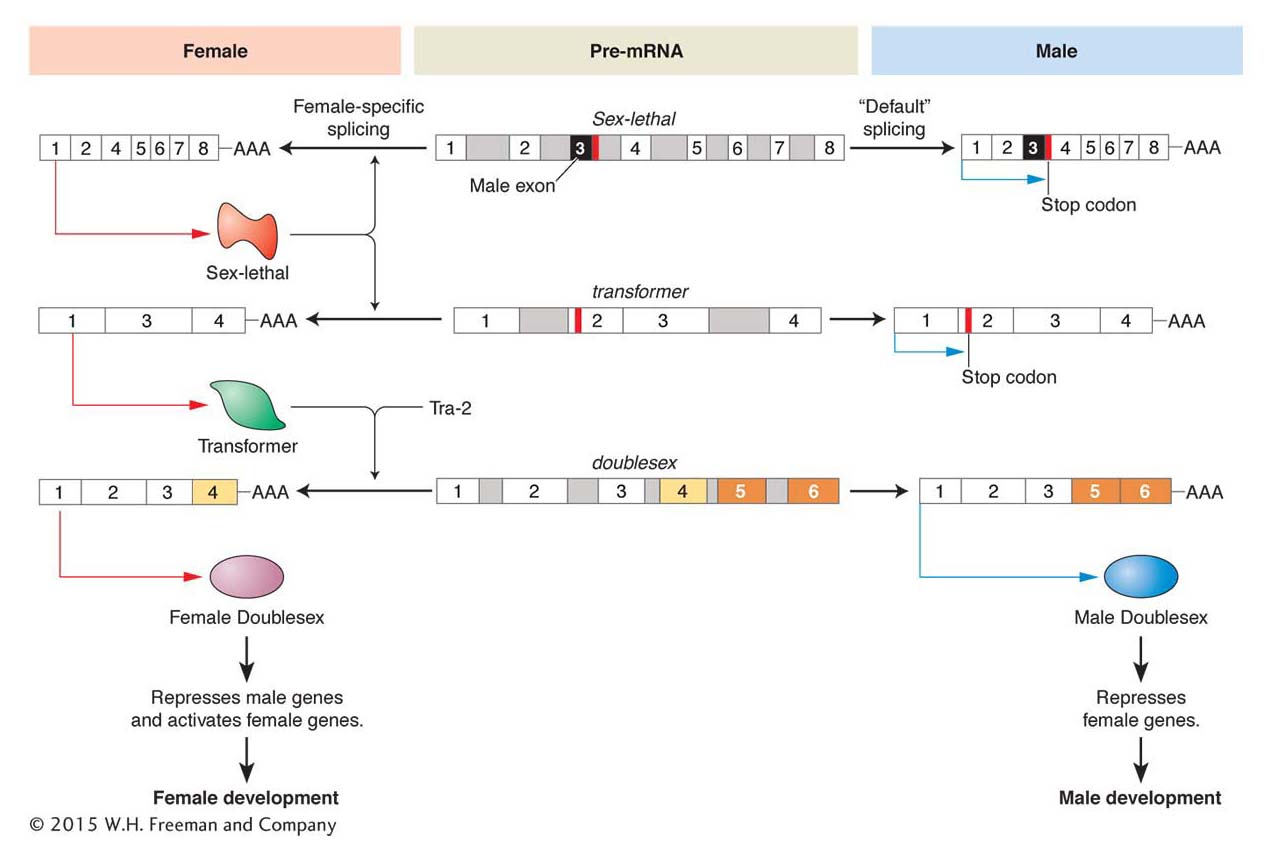
Figure 13-23: A cascade of alternative RNA splicing regulates sex determination in Drosophila
Figure 13-23: Three pre-mRNAs of major Drosophila sex-determining genes are alternatively spliced. The female-specific pathway is shown on the left and the male-specific pathway shown on the right. The pre-mRNAs are identical in both sexes and shown in the middle. In the male Sex-lethal and transformer mRNAs, there are stop codons that terminate translation. These sequences are removed by splicing to produce functional proteins in the female. The Transformer and Tra-2 proteins then splice the female doublesex pre-mRNA to produce the female-specific isoform of the Dsx protein, which differs from the male-specific isoform by the alternative splicing of several exons.
The Tra protein explains how alternative forms of Dsx are expressed, but how is Tra expression itself regulated to differ in females and males? The tra RNA itself is alternatively spliced. In females, a splicing factor encoded by the Sex-lethal (Sxl) gene is present. This splicing factor binds to the tra RNA and prevents a splicing event that would otherwise incorporate an exon that contains a stop codon. In males, no Tra protein is made because this stop codon is present.
The production of the Sex-lethal protein is, in turn, regulated both by RNA splicing and by factors that alter the level of transcription. The level of Sxl transcription is governed by activators on the X chromosome and repressors on the auto-somes. In females, Sxl activation prevails and the Sxl protein is produced, which regulates tra RNA splicing and feeds back to regulate the splicing of Sxl RNA itself. In females, a stop codon is spliced out so that Sxl protein production can continue. However, in males, where no Sxl protein is present, the stop codon is still present in the unspliced Sxl RNA transcript and no Sxl protein can be produced.
This cascade of sex-specific RNA splicing in D. melanogaster illustrates one way that the sex-chromosome genotype leads to different forms of regulatory proteins being expressed in one sex and not the other. Interestingly, the genetic regulation of sex determination differs greatly between animal species, in that sexual genotype can lead to differential expression of regulatory genes through distinctly different paths. However, proteins related to Dsx do play roles in sexual differentiation in a wide variety of animals, including humans. Thus, although there are many ways to generate differential expression of transcription factors, a family of similar proteins appear to underlie much sexual differentiation.
Regulation of mRNA translation and cell lineage in C. elegans
In many animal species, the early development of the embryo entails the partitioning of cells or groups of cells into discrete lineages that will give rise to distinct tissues in the adult. This process is best understood in the nematode worm C. elegans, in which the adult animal is composed of just about 1000 somatic cells (a third of which are nerve cells) and a similar number of germ cells in the gonad. The simple construction, rapid life cycle, and transparency of C. elegans has made it a powerful model for developmental analysis (see the Model Organism box C. elegans). All of this animal’s cell lineages were mapped out in a series of elegant studies led by John Sulston at the Medical Research Council (MRC) Laboratory in Cambridge, England. Systematic genetic screens for mutations that disrupt or extend cell lineages have provided a bounty of information about the genetic control of lineage decisions. C. elegans genetics has been especially important in understanding the role of post-transcriptional regulation at the RNA level, and we will examine two mechanisms here: (1) control of translation by mRNA-binding proteins and (2) miRNA control of gene expression.
Translational control in the early embryo
We first look at how a cell lineage begins. After two cell divisions, the C. elegans embryo contains four cells, called blastomeres. Each cell will begin a distinct lineage, and the descendants of the separate lineages will have different fates. Already at this stage, differences are observed in the proteins present in the four blastomeres. Not surprisingly from what we have learned, many of these proteins are toolkit proteins that determine which genes will be expressed in descendant cells. What is surprising, though, is that the mRNAs encoding some worm toolkit proteins are present in all cells of the early embryo. However, in a specific cell, only some of these mRNAs will be translated into proteins. Thus, in the C. elegans embryo, post-transcriptional regulation is critical for the proper specification of early cell fates. During the very first cell division, polarity within the zygote leads to the partitioning of regulatory molecules to specific embryonic cells. For example, the glp-1 gene encodes a transmembrane receptor protein (related to the Notch receptor of flies and other animals). Although the glp-1 mRNA is present in all cells at the four-cell stage, the GLP-1 protein is translated only in the two anterior cells ABa and ABp (Figure 13-24a). This localized expression of GLP-1 is critical for establishing distinct fates. Mutations that abolish glp-1 function at the four-cell stage alter the fates of ABp and ABa descendants.
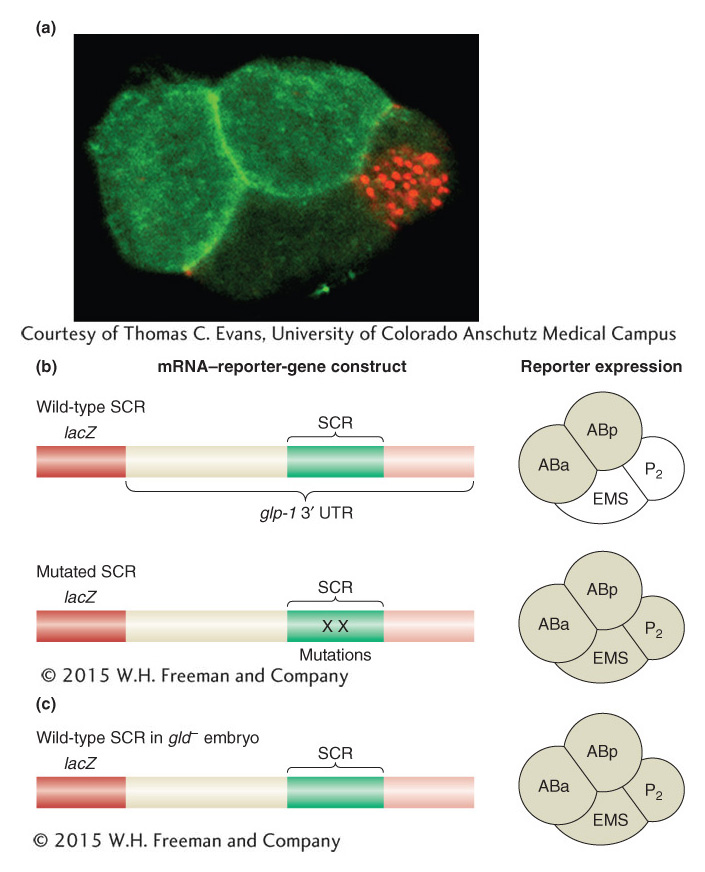
Figure 13-24: mRNA-binding proteins repress mRNA translation to determine cell lineages
Figure 13-24: Translational regulation and cell-lineage decisions in the early C. elegans embryo. (a) At the four-cell stage of the C. elegans embryo, the GLP-1 protein is expressed in two anterior cells (bright green) but not in other cells. Translation of the glp-1 mRNA is regulated by the GLD-1 protein in posterior cells. (b) Fusion of the glp-1 3 ‘ UTR to the lacZ reporter gene leads to reporter expression in the ABa and ABp cells of the four-cell stage of the C. elegans embryo (shaded, right). Mutations in GLD-1-binding sites in the spatial control region (SCR) cause derepression of translation in the EMS and P2 lineages, as does (c) loss of gld function.
[(a) Courtesy of Thomas C. Evans, University of Colorado Anschutz Medical Campus.]
Caenorhabditis elegans
The Nematode Caenorhabditis elegans as a Model for Cell-Lineage-Fate Decisions
In the past 20 years, studies of the nematode worm Caenorhabditis elegans (see Diagram 1) have greatly advanced our understanding of the genetic control of cell-lineage decisions. The transparency and simple construction of this animal led Sydney Brenner to advance its use as a model organism. The adult worm contains about 1000 somatic cells, and researchers, led by John Sulston, have carefully mapped out the entire series of somatic-cell decisions that produce the adult animal.
Diagram 1 An adult hermaphrodite Caenorhabditis elegans, showing various organs.
Some of the lineage decisions, such as the formation of the vulva, have been key models of so-called inductive interactions in development, where signaling between cells induces cell-fate changes and organ formation (see Diagram 2). Exhaustive genetic screens have identified many components participating in signaling and signal transduction in the formation of the vulva.
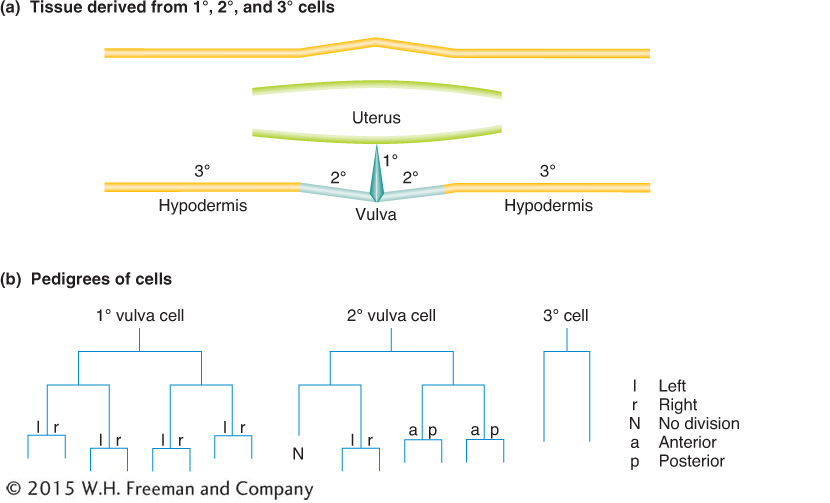
Diagram 2 Production of the vulval-cell lineages. (a) The parts of the vulval anatomy that are occupied by so-called primary (1°), secondary (2°), and tertiary (3°) cells. (b) The lineages or pedigrees of the primary, secondary, and tertiary cells are distinguished by their cell-division patterns.
For some of the embryonic and larval cell divisions, particularly those that will contribute to a worm’s nervous system, a progenitor cell gives rise to two progeny cells, one of which then undergoes programmed cell death. Analysis of mutants in which programmed cell death is aberrant, led by Robert Horvitz, has revealed many components of programmed-cell-death pathways common to most animals. Sydney Brenner, John Sulston, and Robert Horvitz shared the 2002 Nobel Prize in Physiology or Medicine for their pioneering work based on C. elegans.
GLP-1 is localized to the anterior cells by repressing its translation in the posterior cells. The repression of GLP-1 translation requires sequences in the 3′ UTR of the glp-1 mRNA—specifically, a 61-nucleotide region called the spatial control region (SCR). The importance of the SCR has been demonstrated by linking mRNA transcribed from reporter genes to different variants of the SCR. Deletion of this region or mutation of key sites within it causes the reporter gene to be expressed in all four blastomeres of the early embryo (Figure 13-24b).
On the basis of how we have seen transcription controlled, we might guess that one or more proteins bind(s) to the SCR to repress translation of the glp-1 mRNA. To identify these repressor proteins, researchers isolated proteins that bound to the SCR. One protein, GLD-1, binds specifically to a region of the SCR. Furthermore, the GLD-1 protein is enriched in posterior blastomeres, just where the expression of glp-1 is repressed. Finally, when GLD-1 expression is inhibited by using RNA interference, the GLP-1 protein is expressed in posterior blastomeres (Figure 13-24c). This evidence suggests that GLD-1 is a translational repressor protein controlling the expression of glp-1.
The spatial regulation of GLP-1 translation is but one example of translational control in development or by GLD-1. Many other mRNAs are translationally regulated, and GLD-1 binds to other target mRNAs in embryonic and germ-line cells.
KEY CONCEPT
Sequence-specific RNA-binding proteins act through cis-acting RNA sequences to regulate the spatial pattern of protein translation.
miRNA control of developmental timing in C. elegans and other species
Development is a temporally as well as spatially ordered process. When events take place is just as important as where. Mutations in the heterochronic genes of C. elegans have been sources of insight into the control of developmental timing. Mutations in these genes alter the timing of events in cell-fate specification, causing such events to be either reiterated or omitted. Detailed investigation into the products of heterochronic genes led to the discovery of an entirely unexpected mechanism for regulating gene expression, through microRNAs.
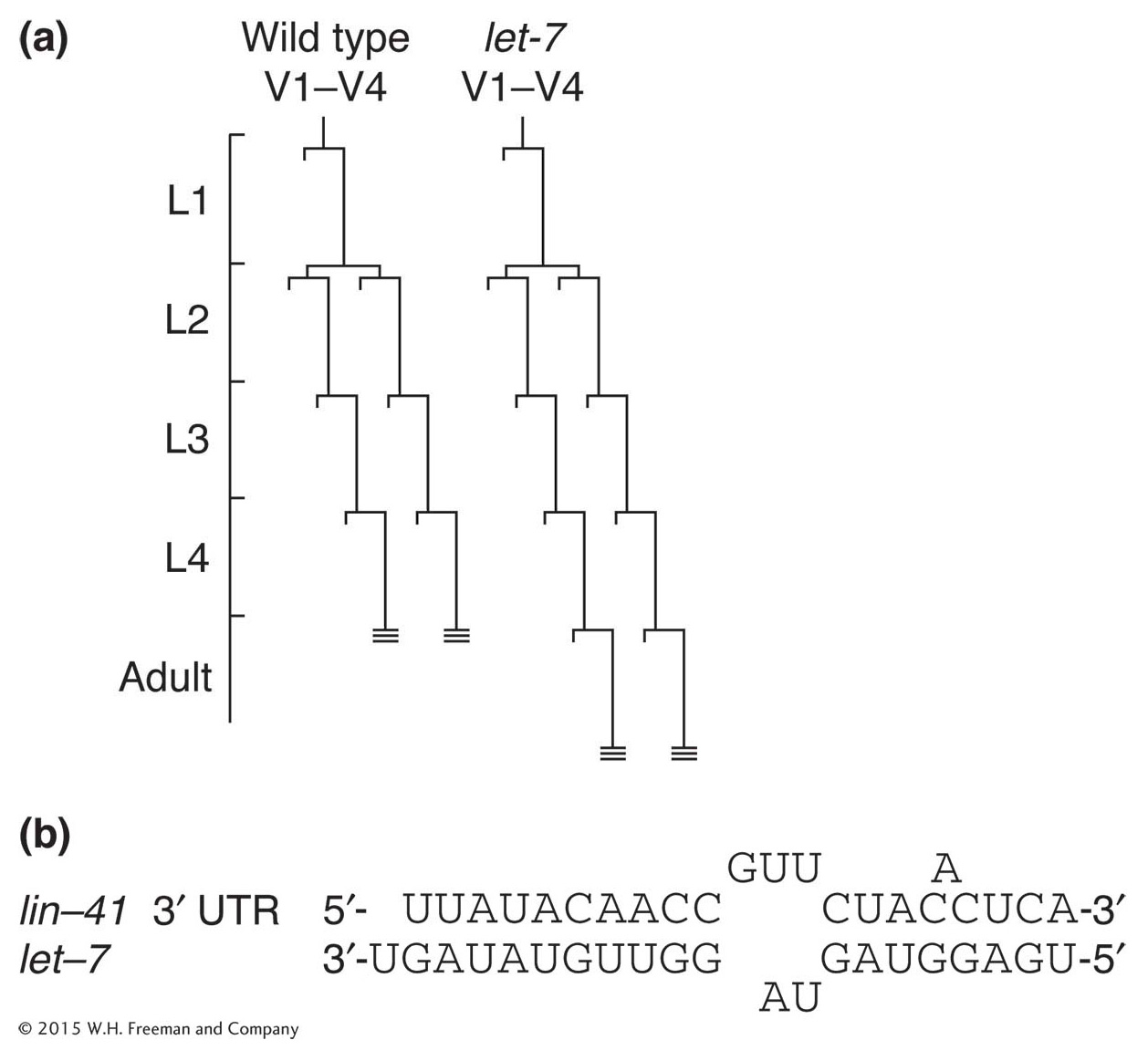
Figure 13-25: A microRNA controls developmental timing
Figure 13-25: Normally, C. elegans develops into an adult after four larval stages, and hypodermal cell lineages conclude their development at L4 (hatched lines at ends of V1-V4 lineages). (a) In let-7 mutants, the transition from the L4 larval stage to adult is delayed and the cell lineages of lateral hypodermal cells (V) are reiterated. (b) let-7 encodes an miRNA that is complementary to sequences in the 3′ UTR of lin-41 mRNA.
Among the first members of this class of regulatory molecules to be discovered in C. elegans is RNA produced by the let-7 gene. The let-7 gene regulates the transition from late-larval to adult cell fates. In let-7 mutants, for example, larval cell fates are reiterated in the adult stage (Figure 13-25a). Conversely, increased let-7 gene dosage causes the precocious specification of adult fates in larval stages.
The let-7 gene does not encode a protein. Instead, it encodes a temporally regulated mature 22-nucleotide RNA that is processed from an approximately 70-nucleotide precursor. The mature RNA is complementary to sequences in 3′ untranslated regions of a variety of developmentally regulated genes, and the binding of the miRNA to these sequences hinders translation of these gene transcripts. One of these target genes, lin-41, also affects the larval-to-adult transition. The lin-41 mutants cause precocious specification of adult cell fates, suggesting that the effect of let-7 overexpression is due at least in part to an effect on lin-41 expression. The let-7 mRNA binds to lin-41 RNA in vitro at several imperfect complementary sites (Figure 13-25b).
The role of miRNAs in C. elegans development extends far beyond let-7. Several hundred miRNAs have been identified, and many target genes have been shown to be miRNA regulated. Moreover, the discovery of this class of regulatory RNAs prompted the search for such genes in other genomes, and, in general, hundreds of candidate miRNA genes have been detected in animal genomes, including those of humans.
Quite surprisingly, the let-7 miRNA gene is widely conserved and found in Drosophila, ascidian, mollusc, annelid, and vertebrate (including human) genomes. The lin-41 gene also is conserved, and evidence suggests that the let-7–lin-41 regulatory interaction also controls the timing of events in the development of other species.
The discoveries of miRNA regulation of developmental genes and of the scope of the miRNA repertoire are fairly recent. Geneticists and other biologists are quite excited about the roles of this class of regulatory molecules in development and physiology, leading to a very vigorous, fast-paced area of new research.




