Bacterial insertion sequences
Insertion sequences, or insertion sequence (IS) elements, are segments of bacterial DNA that can move from one position on a chromosome to a different position on the same chromosome or on a different chromosome. When IS elements appear in the middle of genes, they interrupt the coding sequence and inactivate the expression of that gene. Owing to their size and in some cases the presence of transcription- and translation-termination signals in the IS element, IS elements can also block the expression of other genes in the same operon if those genes are downstream of the insertion site. IS elements were first found in E. coli in the gal operon— a cluster of three genes taking part in the metabolism of the sugar galactose.
Identification of discrete IS elements Several E. coli gal– mutants were found to contain large insertions of DNA into the gal operon. This finding led naturally to the next question: Are the segments of DNA that insert into genes merely random DNA fragments or are they distinct genetic entities? The answer to this question came from the results of hybridization experiments showing that many different insertion mutations are caused by a small set of insertion sequences. These experiments are performed with the use of λdgαl phages that contain the gal– operon from several independently isolated gal mutant strains. Individual phage particles from the strains are isolated, and their DNA is used to synthesize radioactive RNA in vitro. Certain fragments of this RNA are found to hybridize with the DNA from other gal− mutations containing large DNA insertions but not with wild-type DNA. These results were interpreted to mean that independently isolated gal mutants contain the same extra piece of DNA. These particular RNA fragments also hybridize to DNA from other mutants containing IS insertions in other genes, showing that the same bit of DNA can insert in different places in the bacterial chromosome.
Structure of IS elements On the basis of their patterns of cross-hybridization, a number of distinct IS elements have been identified. One sequence, termed IS1, is the 800-bp segment identified in gal. Another sequence, termed IS2, is 1350 bp long. Although IS elements differ in DNA sequence, they have several features in common. For example, all IS elements encode a protein, called a transposase, which is an enzyme required for the movement of IS elements from one site in the chromosome to another. In addition, all IS elements begin and end with short inverted repeat sequences that are required for their mobility. The transposition of IS elements and other mobile genetic elements will be considered later in the chapter.
The genome of the standard wild-type E. coli is rich in IS elements: it contains eight copies of IS1, five copies of IS2, and copies of other less well-studied IS types. Because the same IS elements have identical sequences, they are sites where crossovers may take place. For example, recombination between the F-factor plasmid and the E. coli chromosome to form Hfr strains is the result of a single crossover between an IS1 element located on the plasmid and an IS1 element located on the chromosome (see Figure 5-18). Because there are multiple IS1 elements, the F factor can insert at multiple sites.
KEY CONCEPT
The bacterial genome contains segments of DNA, termed IS elements, that can move from one position on the chromosome to a different position on the same chromosome or on a different chromosome.
Prokaryotic transposons
In Chapter 5, you learned about R plasmids, which carry genes that encode resistance to several antibiotics. These R plasmids (for resistance), also known as R factors, are transferred rapidly on cell conjugation, much like the F factor in E. coli.
The R factors proved to be just the first of many similar F-like factors to be discovered. R factors have been found to carry many different kinds of genes in bacteria. In particular, R factors pick up genes conferring resistance to different antibiotics. How do they acquire their new genetic abilities? It turns out that the drug-resistance genes reside on a mobile genetic element called a transposon (Tn). There are two types of bacterial transposons. Composite transposons contain a variety of genes that reside between two nearly identical IS elements that are oriented in opposite direction (Figure 15-6a) and, as such, form what is called an inverted repeat sequence. Transposase encoded by one of the two IS elements is necessary to catalyze the movement of the entire transposon. An example of a composite transposon is Tn10, shown in Figure 15-6a. Tn10 carries a gene that confers resistance to the antibiotic tetracycline and is flanked by two IS10 elements in opposite orientation. The IS elements that make up composite transposons are not capable of transposing on their own because of mutations in their inverted repeats.
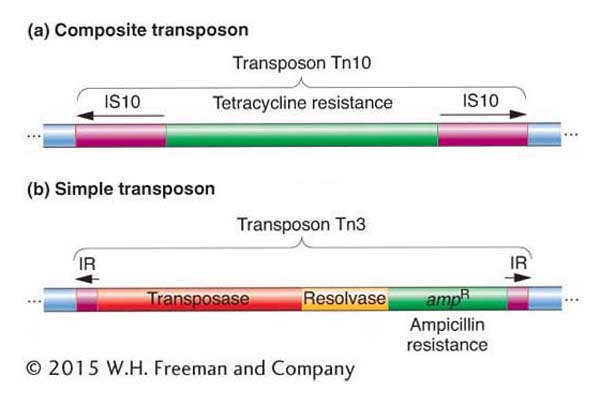
Figure 15-6: Structural features of composite and simple transposons
Figure 15-6: (a) Tn10, an example of a composite transposon. The IS elements are inserted in opposite orientation and form inverted repeats (IRs). Each IS element carries a transposase, but only one is usually functional. (b) Tn3, an example of a simple transposon. Short inverted repeats contain no transposase. Instead, simple transposons encode their own transposase. The resolvase is a protein that promotes recombination and resolves the cointegrates (see Figure 15-9).
Simple transposons also consist of bacterial genes flanked by inverted repeat sequences, but these sequences are short (< 50 bp) and do not encode the transposase enzyme that is necessary for transposition. Thus, their mobility is not due to an association with IS elements. Instead, simple transposons encode their own transposase in the region between the inverted repeat sequences in addition to carrying bacterial genes. An example of a simple transposon is Tn3, shown in Figure 15-6b.
To review, IS elements are short mobile sequences that encode only those proteins necessary for their mobility. Composite transposons and simple transposons contain additional genes that confer new functions to bacterial cells. Whether composite or simple, transposons are usually just called transposons, and different transposons are designated Tn1, Tn2, Tn505, and so forth.
A transposon can jump from a plasmid to a bacterial chromosome or from one plasmid to another plasmid. In this manner, multiple-drug-resistant plasmids are generated. Figure 15-7 is a composite diagram of an R factor, indicating the various places at which transposons can be located. We next consider the question of how such transposition or mobilization events occur.
KEY CONCEPT
Transposons were originally detected as mobile genetic elements that confer drug resistance. Many of these elements consist of IS elements flanking a gene that encodes drug resistance. This organization promotes the spread of drug-resistant bacteria by facilitating movement of the resistance gene from the chromosome of a resistant bacterium to a plasmid that can be conjugated into another (susceptible) bacterial strain.
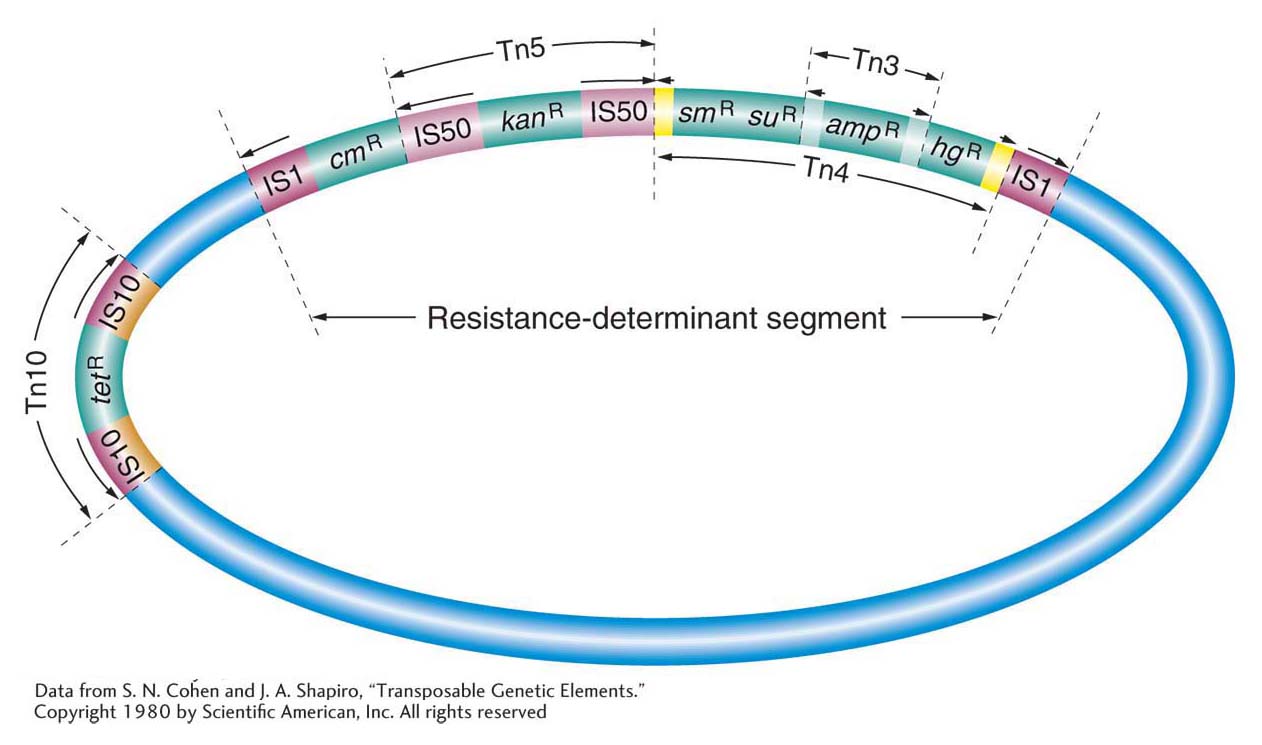
Figure 15-7: An R plasmid may contain several transposons carrying resistance genes
Figure 15-7: A schematic map of a plasmid with several insertions of simple and composite transposons carrying resistance genes. Plasmid sequences are in blue. Genes encoding resistance to the antibiotics tetracycline (tetR), kanamycin (AanR), streptomycin (smR), sulfonamide (suR), and ampicillin (ampR) and to mercury (hgR) are shown. The resistantdeterminant segment can move as a cluster of resistance genes. Tn3 is within Tn4. Each transposon can be transferred independently.
[Data from S. N. Cohen and J. A. Shapiro, “Transposable Genetic Elements.” Copyright 1980 by Scientific American, Inc. All rights reserved]
Mechanism of transposition
As already stated, the movement of a transposable element depends on the action of a transposase. This enzyme plays key roles in the two stages of transposition: excision (leaving) from the original location and inserting into the new location.
Excision from the original location Most transposable elements in prokaryotes (and in eukaryotes) employ one of two mechanisms of transposition, called replicative and conservative (nonreplicative), as illustrated in Figure 15-8. In the replicative pathway (as shown for Tn3), a new copy of the transposable element is generated in the transposition event. The results of the transposition are that one copy appears at the new site and one copy remains at the old site. In the conservative pathway (as shown for Tn10), there is no replication. Instead, the element is excised from the chromosome or plasmid and is integrated into the new site. The conservative pathway is also called "cut and paste".
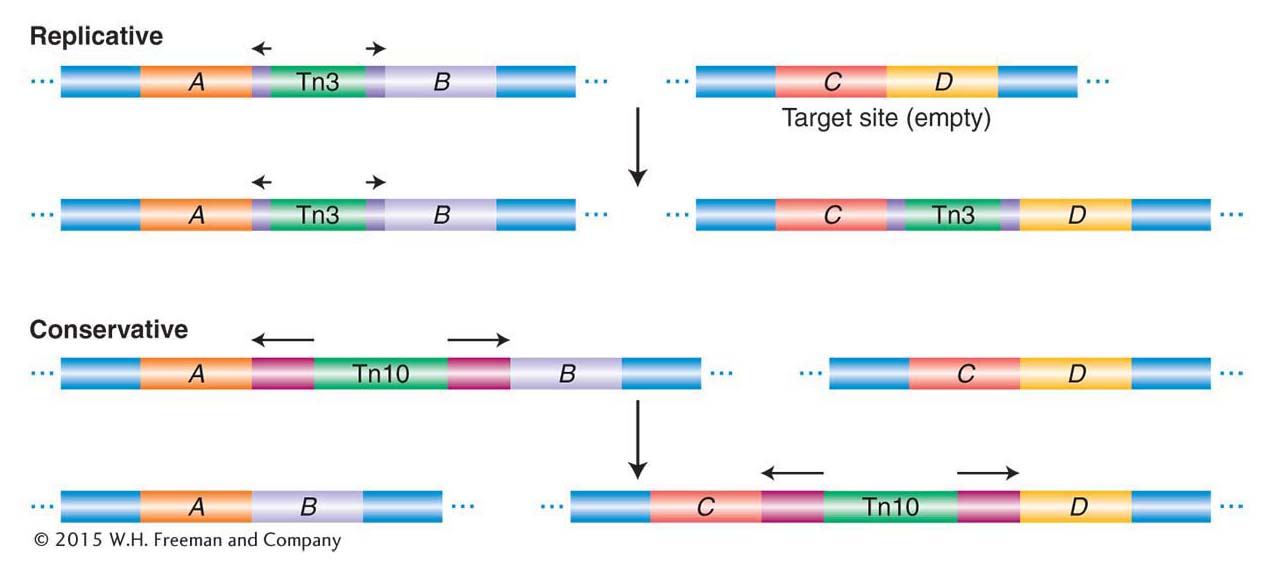
Figure 15-8: Two modes of transposition
Figure 15-8: Mobile-element transposition may be either replicative or conservative. See text for details.
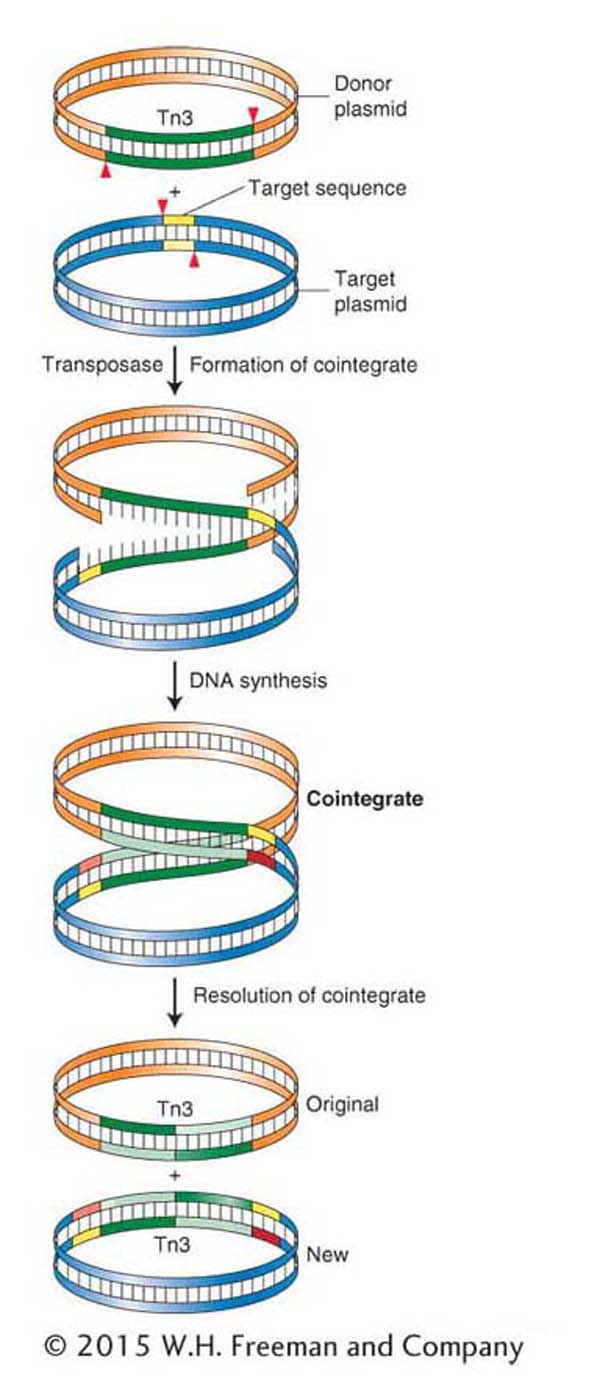
Figure 15-9: Replicative transposition of Tn3
Figure 15-9: Replicative transposition of Tn3 takes place through a cointegrate intermediate. ANIMATED ART: Replicative transposition
Replicative transposition Because this mechanism is a bit complicated, it will be described here in more detail. As Figure 15-8 illustrates, one copy of Tn3 is produced from an initial single copy, yielding two copies of Tn3 altogether.
Figure 15-9 shows the details of the intermediates in the transposition of Tn3 from one plasmid (the donor) to another plasmid (the target). During transposition, the donor and recipient plasmids are temporarily fused together to form a double plasmid. The formation of this intermediate is catalyzed by Tn3-encoded transposase, which makes single-strand cuts at the two ends of Tn3 and staggered cuts at the target sequence and joins the free ends together, forming a fused circle called a cointegrate. The transposable element is duplicated in the fusion event. The cointegrate then resolves by a recombination-like event that turns a cointegrate into two smaller circles, leaving one copy of the transposable element in each plasmid. The result is that one copy remains at the original location of the element, whereas the other is integrated at a new genomic position.
Conservative transposition Some transposons, such as Tn10, excise from the chromosome and integrate into the target DNA. In these cases, the DNA of the element is not replicated, and the element is lost from the site of the original chromosome (see Figure 15-8). Like replicative transposition, this reaction is initiated by the element-encoded transposase, which cuts at the ends of the transposon. However, in contrast with replicative transposition, the transposase cuts the element out of the donor site. It then makes a staggered cut at a target site and inserts the element into the target site. We will revisit this mechanism in more detail in a discussion of the transposition of eukaryotic transposable elements, including the Ac/Ds family of maize.
Insertion into a new location We have seen that transposase continues to play an important role in insertion. In one of the first steps of insertion, the transposase makes a staggered cut in the target-site DNA (not unlike the staggered breaks catalyzed by restriction endonucleases in the sugar–phosphate backbone of DNA). Figure 15-10 shows the steps in the insertion of a generic transposable element. In this case, the transposase makes a five-base-pair staggered cut. The transposable element inserts between the staggered ends, and the host DNA repair machinery (see Chapter 16) fills in the gap opposite each single-strand overhang by using the bases in the overhang as a template. There are now two duplicate sequences, each five base pairs in length, at the sites of the former overhangs. These sequences are called a target-site duplication. Virtually all transposable elements (in both prokaryotes and eukaryotes) are flanked by a target-site duplication, indicating that all use a mechanism of insertion similar to that shown in Figure 15-10. What differs is the length of the duplication; a particular type of transposable element has a characteristic length for its target-site duplication—as small as two base pairs for some elements. It is important to keep in mind that the transposable elements have inverted repeats at their ends and that the inverted repeats are flanked by the target-site duplication—which is a direct repeat.
KEY CONCEPT
In prokaryotes, transposition occurs by at least two different pathways. Some transposable elements can replicate a copy of the element into a target site, leaving one copy behind at the original site. In other cases, transposition consists of the direct excision of the element and its reinsertion into a new site.
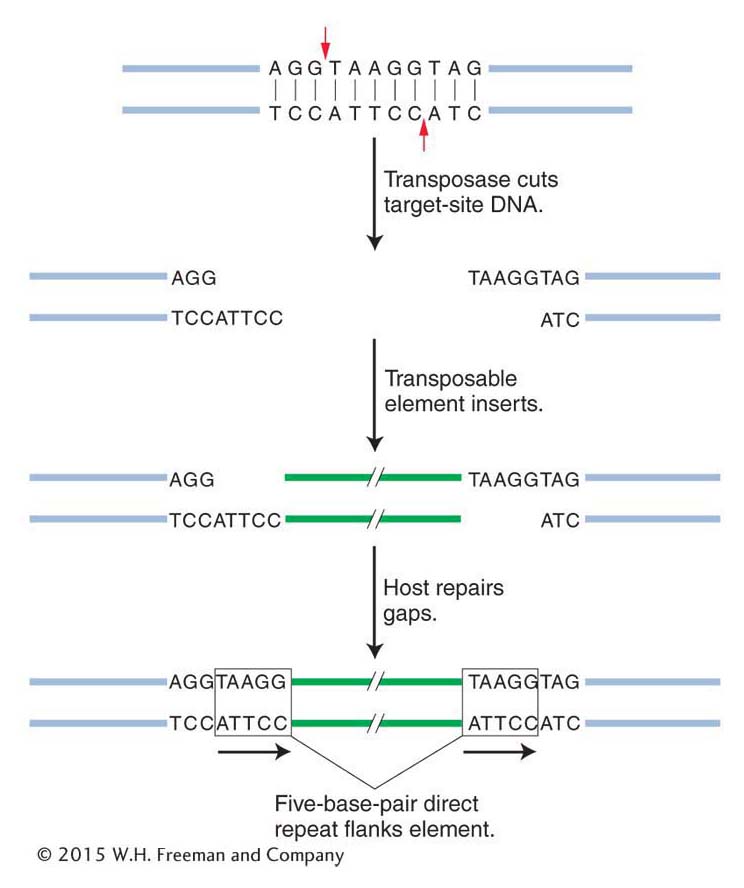
Figure 15-10: An inserted element is flanked by a short repeat
Figure 15-10: A short sequence of DNA is duplicated at the transposon insertion site. The recipient DNA is cleaved at staggered sites (a 5-bp staggered cut is shown), leading to the production of two copies of the five-base-pair sequence flanking the inserted element.




