17.2 Changes in Chromosome Structure
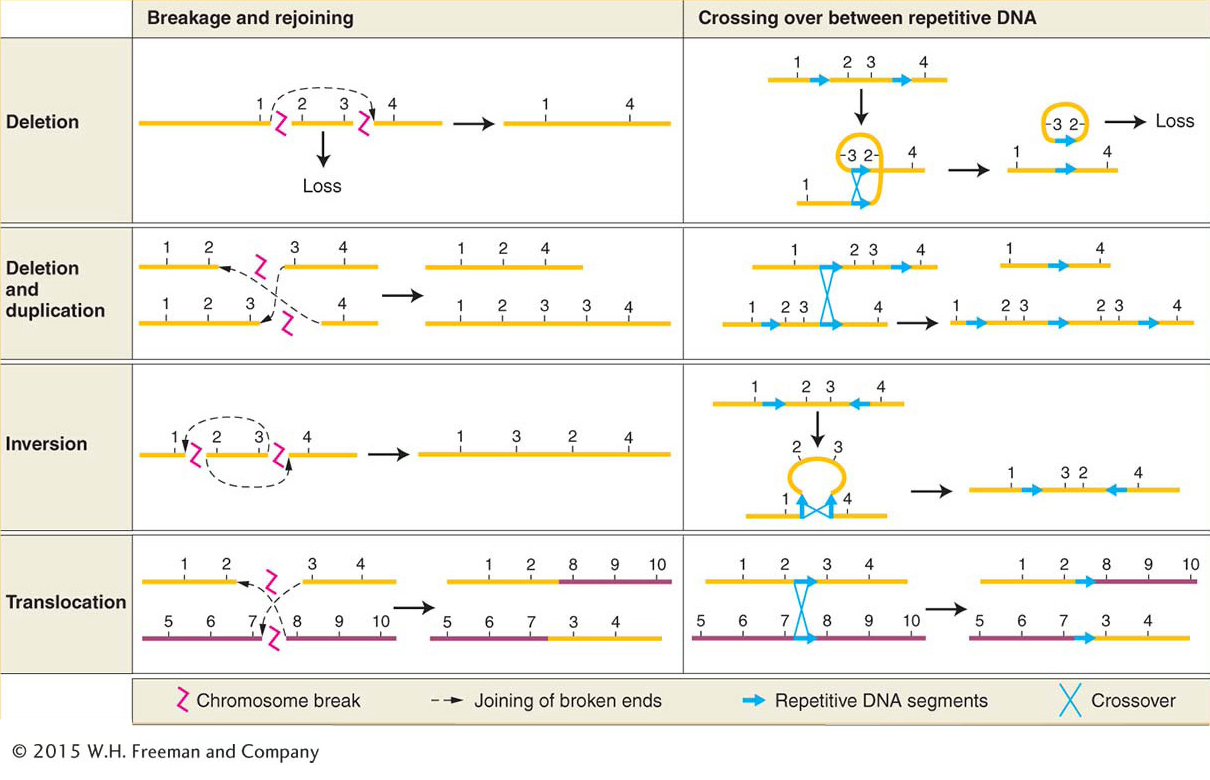
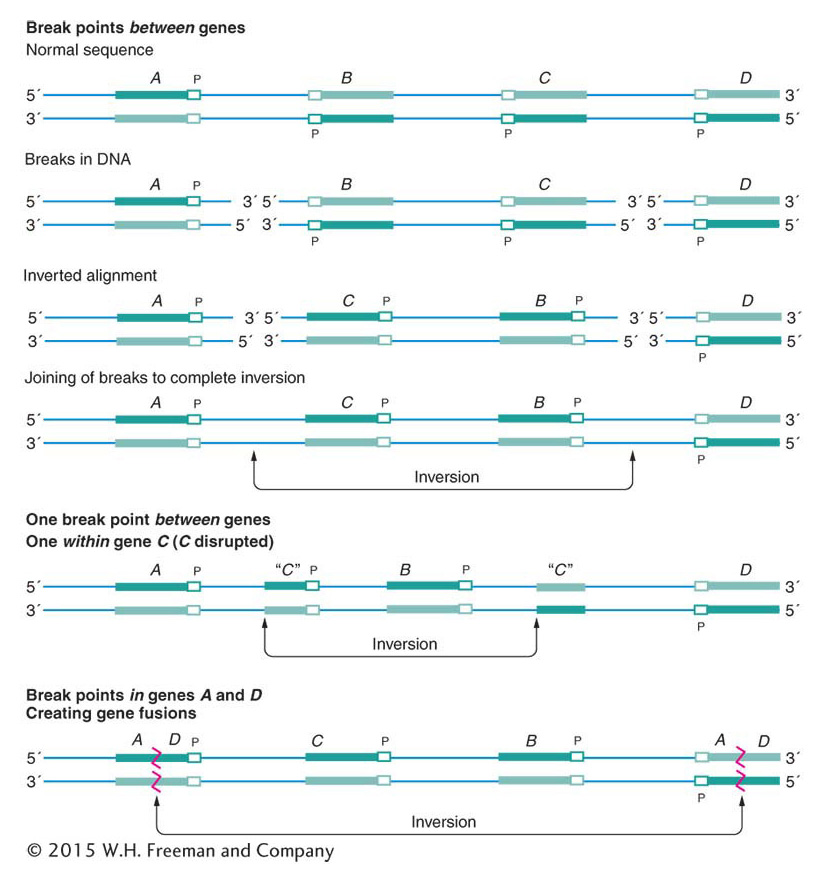
Changes in chromosome structure, called rearrangements, encompass several major classes of events. A chromosome segment can be lost, constituting a deletion, or doubled, to form a duplication. The orientation of a segment within the chromosome can be reversed, constituting an inversion. Or a segment can be moved to a different chromosome, constituting a translocation. DNA breakage is a major cause of each of these events. Both DNA strands must break at two different locations, followed by a rejoining of the broken ends to produce a new chromosomal arrangement (Figure 17-19, left side). Chromosomal rearrangements by breakage can be induced artificially by using ionizing radiation. This kind of radiation, particularly X rays and gamma rays, is highly energetic and causes numerous double-
635
To understand how chromosomal rearrangements are produced by breakage, several points should be kept in mind:
Each chromosome is a single double-
stranded DNA molecule. The first event in the production of a chromosomal rearrangement is the generation of two or more double-
stranded breaks in the chromosomes of a cell (see Figure 17-19, top row at left). Double-
stranded breaks are potentially lethal, unless they are repaired. Repair systems in the cell correct the double-
stranded breaks by joining broken ends back together (see Chapter 16 for a detailed discussion of DNA repair). If the two ends of the same break are rejoined, the original DNA order is restored. If the ends of two different breaks are joined, however, one result is one or another type of chromosomal rearrangement.
The only chromosomal rearrangements that survive meiosis are those that produce DNA molecules that have one centromere and two telomeres. If a rearrangement produces a chromosome that lacks a centromere, such an acentric chromosome will not be dragged to either pole at anaphase of mitosis or meiosis and will not be incorporated into either progeny nucleus. Therefore, acentric chromosomes are not inherited. If a rearrangement produces a chromosome with two centromeres (a dicentric chromosome), it will often be pulled simultaneously to opposite poles at anaphase, forming an anaphase bridge. Anaphase-
bridge chromosomes typically will not be incorporated into either progeny cell. If a chromosome break produces a chromosome lacking a telomere, that chromosome cannot replicate properly. Recall from Chapter 7 that telomeres are needed to prime proper DNA replication at the ends (see Figure 7- 26). 636
If a rearrangement duplicates or deletes a segment of a chromosome, gene balance may be affected. The larger the segment that is lost or duplicated, the more likely it is that gene imbalance will cause phenotypic abnormalities.
Another important cause of rearrangements is crossing over between repetitive (duplicated) DNA segments. This type of crossing over is termed nonallelic homologous recombination (NAHR). In organisms with repeated DNA sequences within one chromosome or on different chromosomes, there is ambiguity about which of the repeats will pair with each other at meiosis. If sequences pair up that are not in the same relative positions on the homologs, crossing over can produce aberrant chromosomes. Deletions, duplications, inversions, and translocations can all be produced by such crossing over (see Figure 17-19, right side).
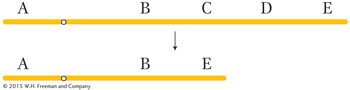
There are two general types of rearrangements: unbalanced and balanced. Unbalanced rearrangements change the gene dosage of a chromosome segment. As with aneuploidy for whole chromosomes, the loss of one copy of a segment or the addition of an extra copy can disrupt normal gene balance. The two simple classes of unbalanced rearrangements are deletions and duplications. A deletion is the loss of a segment within one chromosome arm and the juxtaposition of the two segments on either side of the deleted segment, as in this example, which shows loss of segment C–
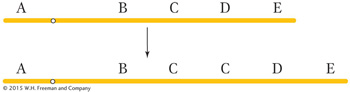
A duplication is the repetition of a segment of a chromosome arm. In the simplest type of duplication, the two segments are adjacent to each other (a tandem duplication), as in this duplication of segment C:
However, the duplicate segment can end up at a different position on the same chromosome or even on a different chromosome.
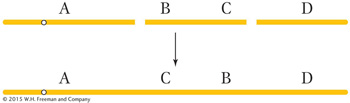
Balanced rearrangements change the chromosomal gene order but do not remove or duplicate any DNA. The two simple classes of balanced rearrangements are inversions and reciprocal translocations. An inversion is a rearrangement in which an internal segment of a chromosome has been broken twice, flipped 180 degrees, and rejoined.
637
A reciprocal translocation is a rearrangement in which two nonhomologous chromosomes are each broken once, creating acentric fragments, which then trade places:
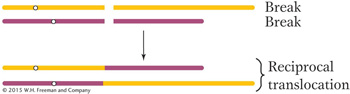
Sometimes the DNA breaks that precede the formation of a rearrangement occur within genes. When they do, they disrupt gene function because part of the gene moves to a new location and no complete transcript can be made. In addition, the DNA sequences on either side of the rejoined ends of a rearranged chromosome are sequences that are not normally juxtaposed. Sometimes the junction occurs in such a way that fusion produces a nonfunctional hybrid gene composed of parts of two other genes.
The following sections consider the properties of these balanced and unbalanced rearrangements.
Deletions
A deletion is simply the loss of a part of one chromosome arm. The process of deletion requires two chromosome breaks to cut out the intervening segment. The deleted fragment has no centromere; consequently, it cannot be pulled to a spindle pole in cell division and is lost. The effects of deletions depend on their size. A small deletion within a gene, called an intragenic deletion, inactivates the gene and has the same effect as that of other null mutations of that gene. If the homozygous null phenotype is viable (as, for example, in human albinism), the homozygous deletion also will be viable. Intragenic deletions can be distinguished from mutations caused by single nucleotide changes because genes with such deletions never revert to wild type.
For most of this section, we will be dealing with multigenic deletions, in which several to many genes are missing. The consequences of these deletions are more severe than those of intragenic deletions. If such a deletion is made homotyzygous by inbreeding (that is, if both homologs have the same deletion), the combination is always lethal. This fact suggests that all regions of the chromosomes are essential for normal viability and that complete elimination of any segment from the genome is deleterious. Even an individual organism heterozygous for a multigenic deletion—
KEY CONCEPT
The lethality of large heterozygous deletions can be explained by gene imbalance and the expression of deleterious recessives.
Small deletions are sometimes viable in combination with a normal homolog. Such deletions may be identified by examining meiotic chromosomes under the microscope. The failure of the corresponding segment on the normal homolog to pair creates a visible deletion loop (Figure 17-20a). In Drosophila, deletion loops are also visible in the polytene chromosomes. These chromosomes are found in the cells of salivary glands and other specific tissues of certain insects. In these cells, the homologs pair and replicate many times, and so each chromosome is represented by a thick bundle of replicates. These polytene chromosomes are easily visible, and each has a set of dark-
638
Another clue to the presence of a deletion is that the deletion of a segment on one homolog sometimes unmasks recessive alleles present on the other homolog, leading to their unexpected expression. Consider, for example, the deletion shown in the following diagram:
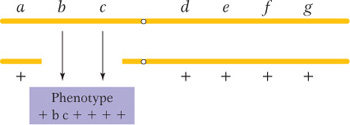
If there is no deletion, none of the seven recessive alleles is expected to be expressed; however, if b and c are expressed, then a deletion spanning the b+ and c+ genes has probably occurred on the other homolog. Because recessive alleles seem to be showing dominance in such cases, the effect is called pseudodominance.
In the reverse case—
KEY CONCEPT
Deletions can be recognized by deletion loops and pseudodominance.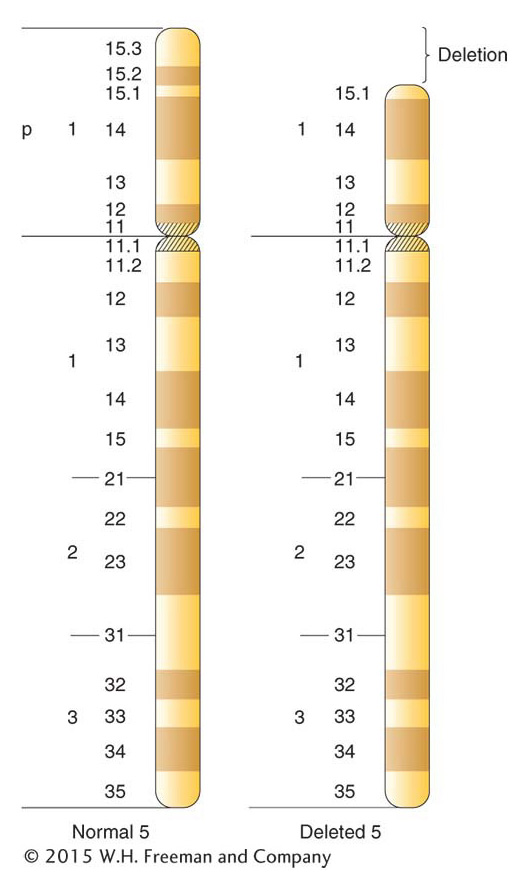
Clinicians regularly find deletions in human chromosomes. The deletions are usually small, but they do have adverse effects, even though heterozygous. Deletions of specific human chromosome regions cause unique syndromes of phenotypic abnormalities. One example is cri du chat syndrome, caused by a heterozygous deletion of the tip of the short arm of chromosome 5 (Figure 17-22). The specific bands deleted in cri du chat syndrome are 5p15.2 and 5p15.3, the two most distal bands identifiable on 5p. (The short and long arms of human chromosomes are traditionally called p and q, respectively.) The most characteristic phenotype in the syndrome is the one that gives it its name, the distinctive catlike mewing cries made by affected infants. Other manifestations of the syndrome are microencephaly (abnormally small head) and a moonlike face. Like syndromes caused by other deletions, cri du chat syndrome includes mental retardation. Fatality rates are low, and many persons with this deletion reach adulthood.
Another instructive example is Williams syndrome. This syndrome is autosomal dominant and is characterized by unusual development of the nervous system and certain external features. Williams syndrome is found at a frequency of about 1 in 10,000 people. Patients often have pronounced musical or singing ability. The syndrome is almost always caused by a 1.5-
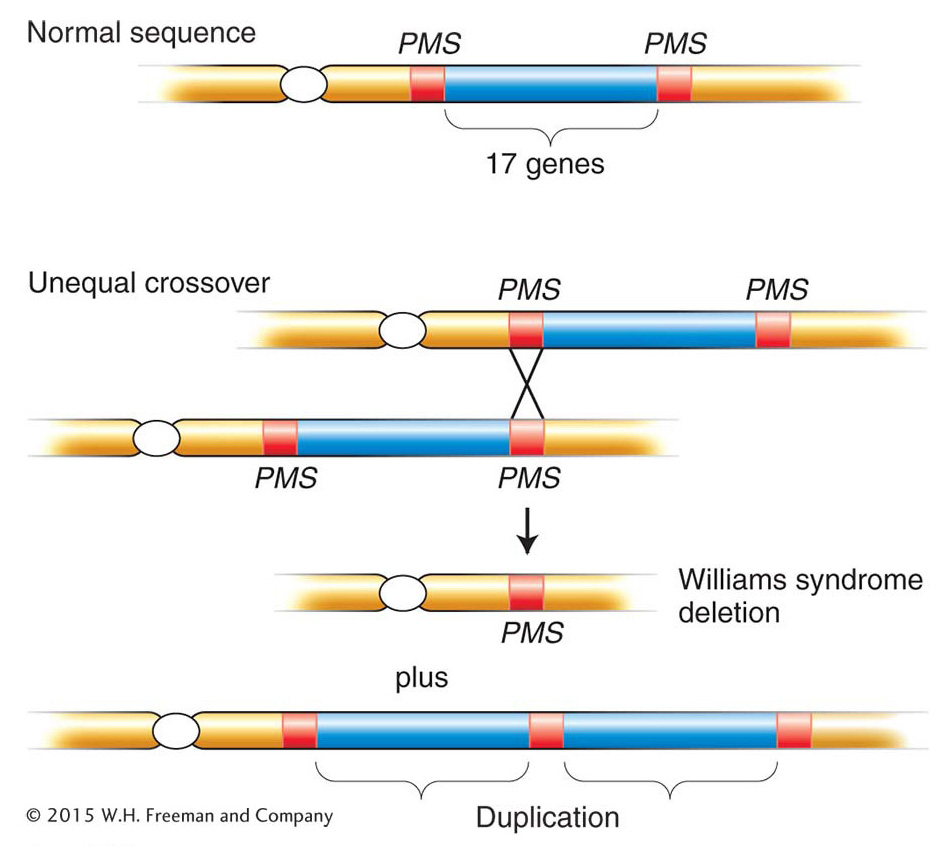
639
Most human deletions, such as those that we have just considered, arise spontaneously in the gonads of a normal parent of an affected person; thus, no signs of the deletions are usually found in the chromosomes of the parents. Less commonly, deletion-
Animals and plants show differences in the survival of gametes or offspring that bear deletions. A male animal with a deletion in one chromosome produces sperm carrying one or the other of the two chromosomes in approximately equal numbers. These sperm seem to function to some extent regardless of their genetic content. In diploid plants, on the other hand, the pollen produced by a deletion heterozygote is of two types: functional pollen carrying the normal chromosome and nonfunctional (aborted) pollen carrying the deficient homolog. Thus, pollen cells seem to be sensitive to changes in the amount of chromosomal material, and this sensitivity might act to weed out deletions. This effect is analogous to the sensitivity of pollen to whole-
640
Duplications
The processes of chromosome mutation sometimes produce an extra copy of some chromosome region. The duplicate regions can be located adjacent to each other—
Synthetic duplications of known coverage can be used for gene mapping. In haploids, for example, a chromosomally normal strain carrying a new recessive mutation m may be crossed with strains bearing a number of duplication-
641
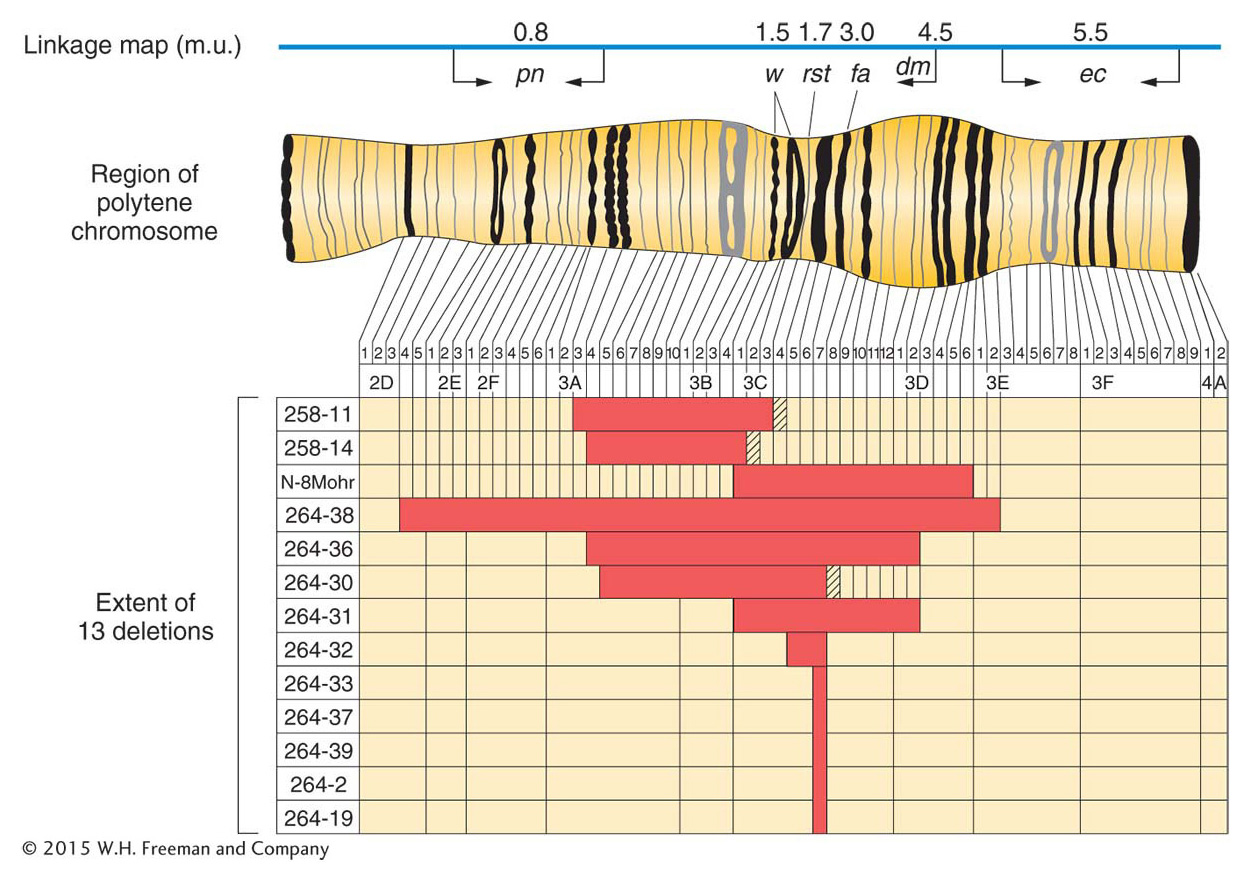
Analyses of genome DNA sequences have revealed a high level of duplications in humans and in most of the model organisms. Simple sequence repeats, which are extensive throughout the genome and useful as molecular markers in mapping, were discussed in earlier chapters. However, another class of duplications is based on duplicated units that are much bigger than the simple sequence repeats. Duplications in this class are termed segmental duplications. The duplicated units in segmental duplications range from 10 to 50 kilobases in length and encompass whole genes and the regions in between. The extent of segmental duplications is shown in Figure 17-24, in which most of the duplications are dispersed, but there are some tandem cases. Another property shown in Figure 17-24 is that the dispersion of the duplicated units is mostly within the same chromosome, not between chromosomes. The origin of segmental duplications is still not known.
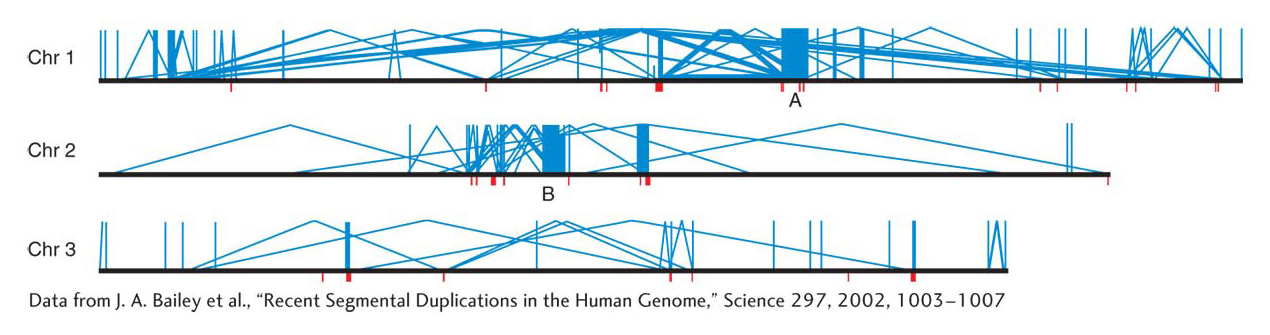
Segmental duplications are thought to have an important role as substrates for nonallelic homologous recombination, as shown in Figure 17-19. Crossing over between segmental duplications can lead to various chromosomal rearrangements. These rearrangements seem to have been important in evolution, inasmuch as some major inversions that are key differences between human and ape sequences have almost certainly come from NAHR (non-
We have seen that, in some organisms such as polyploids, the present-
642
Inversions
We have seen that, to create an inversion, a segment of a chromosome is cut out, flipped, and reinserted. Inversions are of two basic types. If the centromere is outside the inversion, the inversion is said to be paracentric. Inversions spanning the centromere are pericentric.
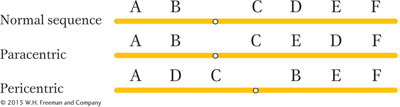
Because inversions are balanced rearrangements, they do not change the overall amount of genetic material, and so they do not result in gene imbalance. Individuals with inversions are generally normal, if there are no breaks within genes. A break that disrupts a gene produces a mutation that may be detectable as an abnormal phenotype. If the gene has an essential function, then the break point acts as a lethal mutation linked to the inversion. In such a case, the inversion cannot be bred to homozygosity. However, many inversions can be made homozygous, and, furthermore, inversions can be detected in haploid organisms. In these cases, the break points of the inversion are clearly not in essential regions. Some of the possible consequences of inversion at the DNA level are shown in Figure 17-26.
643
ANIMATED ART: Chromosome rearrangements: formation of paracentric inversions
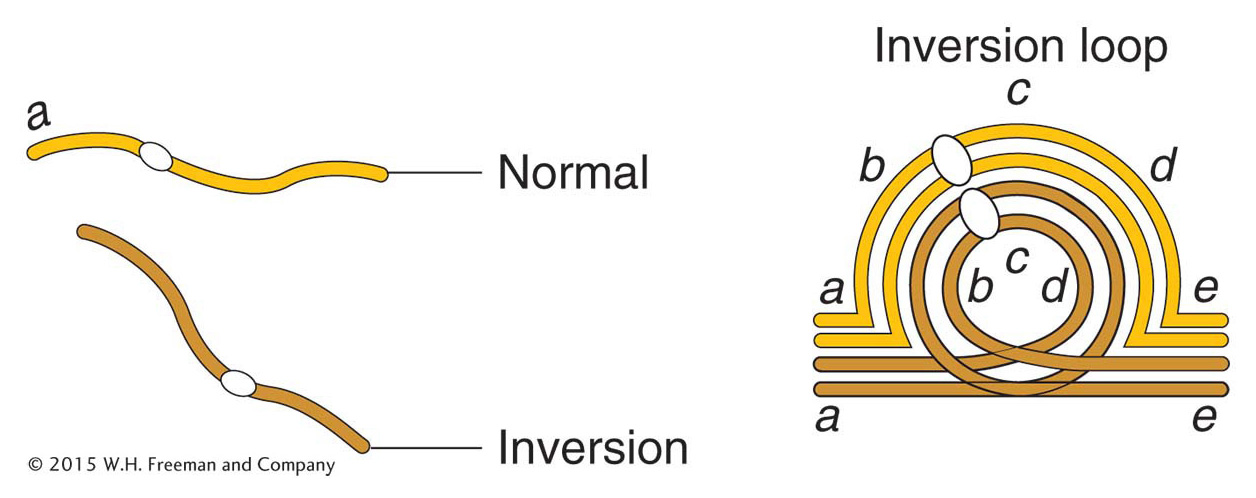
Most analyses of inversions are carried out on diploid cells that contain one normal chromosome set plus one set carrying the inversion. This type of cell is called an inversion heterozygote, but note that this designation does not imply that any gene locus is heterozygous; rather, it means that one normal and one abnormal chromosome set are present. The location of the inverted segment can often be detected microscopically. In meiosis, one chromosome twists once at the ends of the inversion to pair with its untwisted homolog; in this way, the paired homologs form a visible inversion loop (Figure 17-27).
ANIMATED ART: Chromosome rearrangements: meiotic behavior of paracentric inversions
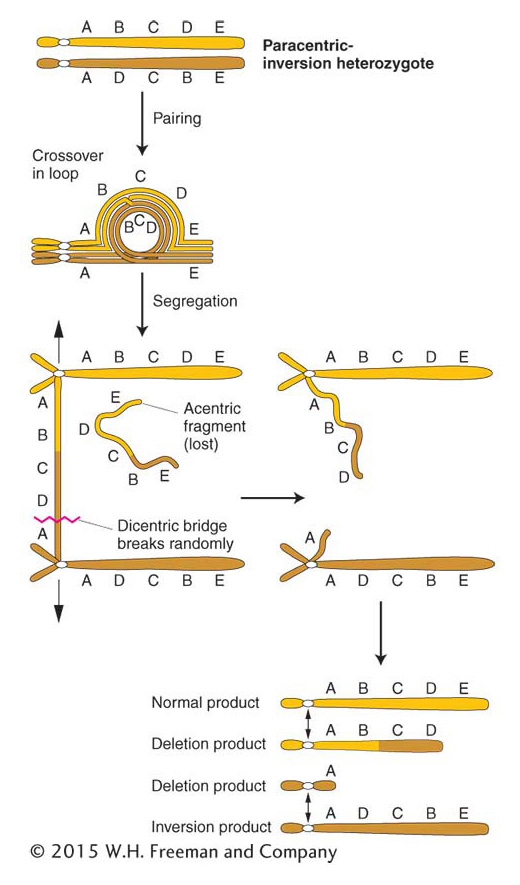
In a paracentric inversion, crossing over within the inversion loop at meiosis connects homologous centromeres in a dicentric bridge while also producing an acentric fragment (Figure 17-28). Then, as the chromosomes separate in anaphase I, the centromeres remain linked by the bridge. The acentric fragment cannot align itself or move; consequently, it is lost. Tension eventually breaks the dicentric bridge, forming two chromosomes with terminal deletions. Either the gametes containing such chromosomes or the zygotes that they eventually form will probably be inviable. Hence, a crossover event, which normally generates the recombinant class of meiotic products, is instead lethal to those products. The overall result is a drastically lower frequency of viable recombinants. In fact, for genes within the inversion, the recombinant frequency is close to zero. (It is not exactly zero because rare double crossovers between only two chromatids are viable.) For genes flanking the inversion, the RF is reduced in proportion to the size of the inversion because, for a longer inversion, there is a greater probability of a crossover occurring within it and producing an inviable meiotic product.
644
In a heterozygous pericentric inversion, the net genetic effect is the same as that of a paracentric inversion—
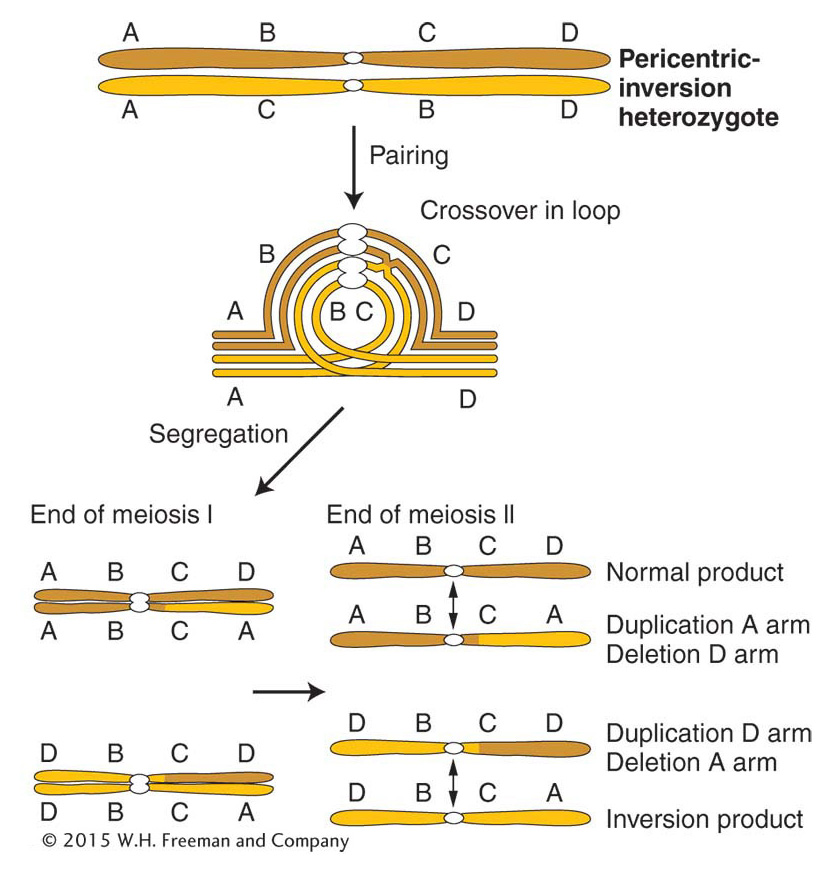
Inversions affect recombination in another way, too. Inversion heterozygotes often have mechanical pairing problems in the region of the inversion. The inversion loop causes a large distortion that can extend beyond the loop itself. This distortion reduces the opportunity for crossing over in the neighboring regions.
Let us consider an example of the effects of an inversion on recombinant frequency. A wild-
|
250 |
wild type |
+ + / dp cn |
|
246 |
dumpy cinnabar |
dp cn / dp cn |
|
5 |
dumpy |
dp + / dp cn |
|
7 |
cinnabar |
+ cn / dp cn |
645
In this cross, which is effectively a dihybrid testcross, 45 percent of the progeny are expected to be dumpy or cinnabar (they constitute the crossover classes), but only 12 of 508, about 2 percent, are obtained. Something is reducing crossing over in this region, and a likely explanation is an inversion spanning most of the dp–
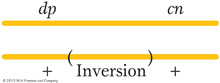
Pericentric inversions also can be detected microscopically through new arm ratios. Consider the following pericentric inversion:
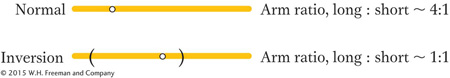
Note that the length ratio of the long arm to the short arm has been changed from about 4:1 to about 1:1 by the inversion. Paracentric inversions do not alter the arm ratio, but they may be detected microscopically by observing changes in banding or other chromosomal landmarks, if available.
KEY CONCEPT
The main diagnostic features of heterozygous inversions are inversion loops, reduced recombinant frequency, and reduced fertility because of unbalanced or deleted meiotic products.In some model experimental systems, notably Drosophila and the nematode Caenorhabditis elegans, inversions are used as balancers. A balancer chromosome contains multiple inversions; so, when it is combined with the corresponding wildtype chromosome, there can be no viable crossover products. In some analyses, it is important to keep stock with all the alleles on one chromosome together. The geneticist creates individuals having genomes that combine such a chromosome with a balancer. This combination eliminates crossovers, and so only parental combinations appear in the progeny. For convenience, balancer chromosomes are marked with a dominant morphological mutation. The marker allows the geneticist to track the segregation of the entire balancer or its normal homolog by noting the presence or absence of the marker.
Reciprocal translocations
There are several types of translocations, but here we consider only reciprocal translocations, the simplest type. Recall that, to form a reciprocal translocation, two chromosomes trade acentric fragments created by two simultaneous chromosome breaks. As with other rearrangements, meiosis in heterozygotes having two translocated chromosomes and their normal counterparts produces characteristic configurations. Figure 17-30 illustrates meiosis in an individual that is heterozygous for a reciprocal translocation. Note the cross-
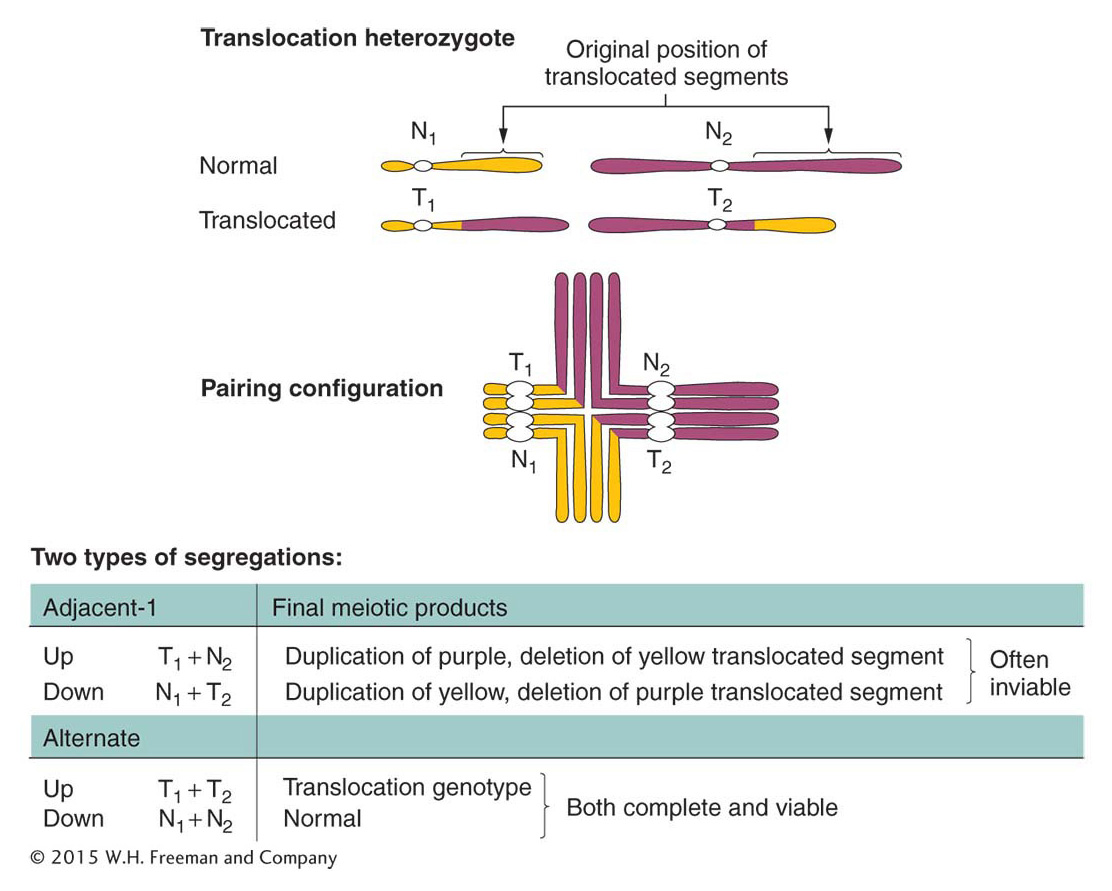
ANIMATED ART: Chromosome rearrangements: reciprocal translocation
646

Adjacent-
Remember that heterozygotes for inversions also may show some reduction in fertility but by an amount dependent on the size of the affected region. The precise 50 percent reduction in viable gametes or zygotes is usually a reliable diagnostic clue for a translocation.
Genetically, genes on translocated chromosomes act as though they are linked if their loci are close to the translocation break point. Figure 17-32 shows a translocation heterozygote that has been established by crossing an a/a; b/b individual with a translocation homozygote bearing the wild-
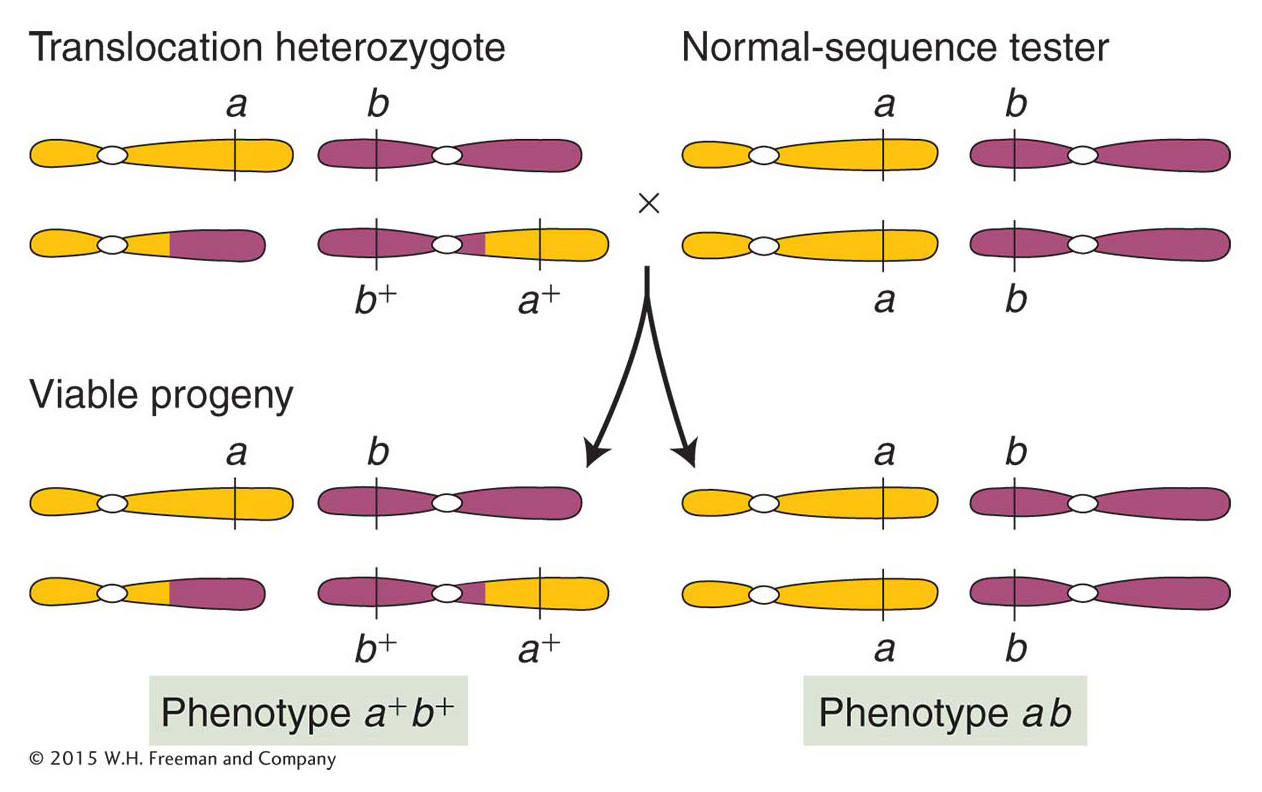
ANIMATED ART: Chromosome rearrangements: pseudolinkage of genes
647
KEY CONCEPT
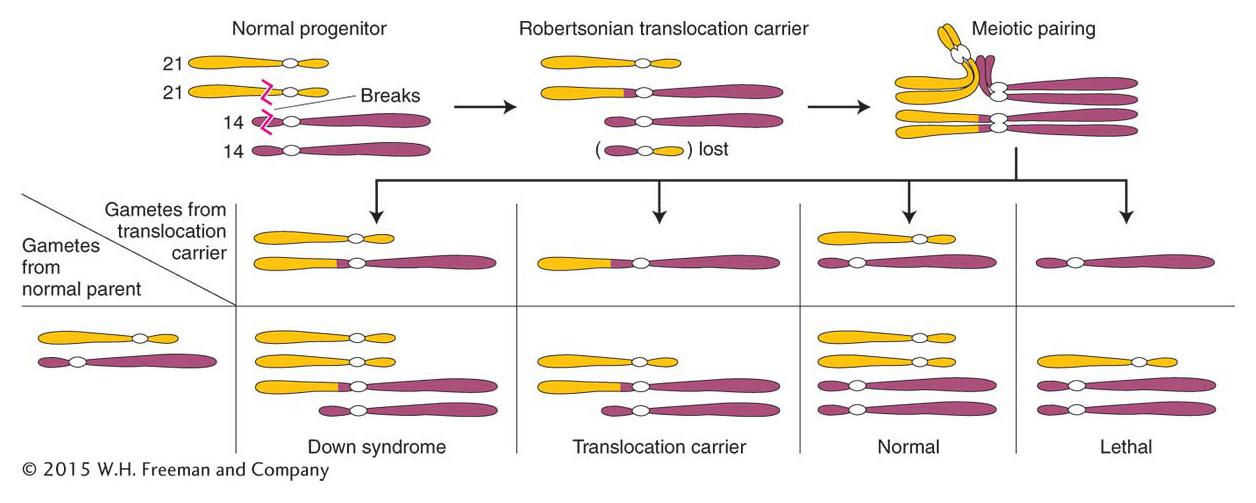
Heterozygous reciprocal translocations are diagnosed genetically by semisterility and by the apparent linkage of genes whose normal loci are on separate chromosomes.Robertsonian translocations
Let’s return to the family with the Down syndrome child, introduced at the beginning of the chapter. The birth can indeed be a coincidence—
648
Applications of inversions and translocations
Inversions and translocations have proved to be useful genetic tools; some examples of their uses follow.
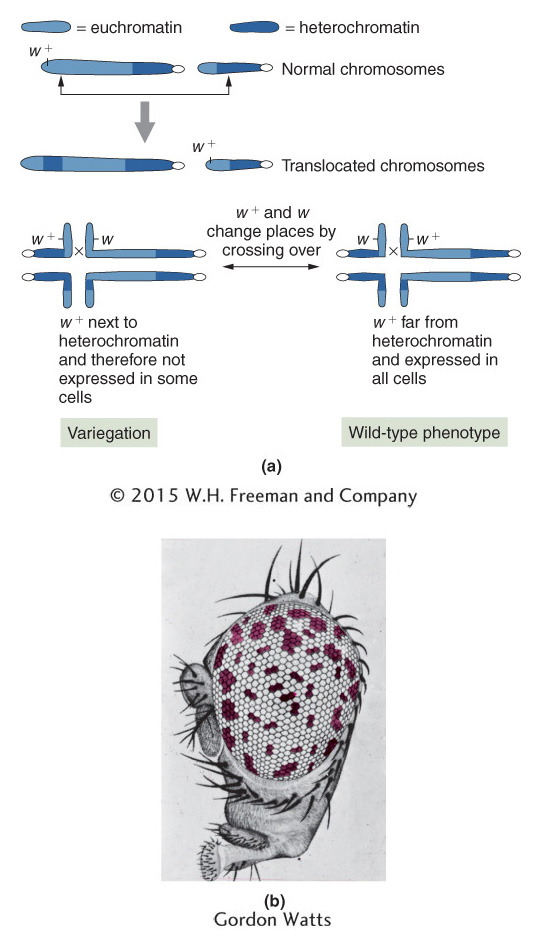
Gene mapping Inversions and translocations are useful for the mapping and subsequent isolation of specific genes. The gene for human neurofibromatosis was isolated in this way. The critical information came from people who not only had the disease, but also carried chromosomal translocations. All the translocations had one break point in common, in a band close to the centromere of chromosome 17. Hence, this band appeared to be the locus of the neurofibromatosis gene, which had been disrupted by the translocation break point. Subsequent analysis showed that the chromosome 17 break points were not at identical positions; however, because they must have been within the gene, the range of their positions revealed the segment of the chromosome that constituted the neurofibromatosis gene. The isolation of DNA fragments from this region eventually led to the recovery of the gene itself.
Synthesizing specific duplications or deletions Translocations and inversions are routinely used to delete or duplicate specific chromosome segments. Recall, for example, that pericentric inversions as well as translocations generate products of meiosis that contain a duplication and a deletion (see Figures 17-29 and 17-30). If the duplicated or the deleted segment is very small, then the duplication-
Another approach to creating duplications uses unidirectional insertional translocations, in which a segment of one chromosome is removed and inserted into another. In an insertional-
Position-
649
Rearrangements and cancer
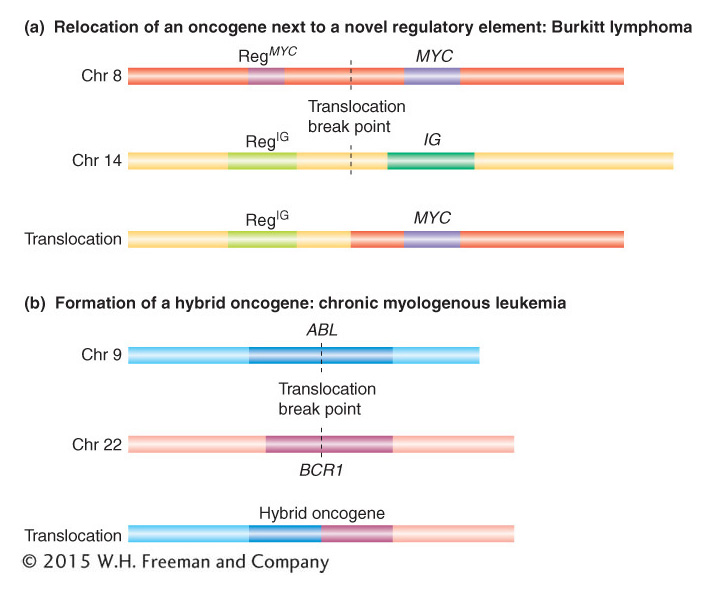
Cancer is a disease of abnormal cell proliferation. As a result of some insult inflicted on it, a cell of the body divides out of control to form a population of cells called a cancer. A localized knot of proliferated cells is called a tumor, whereas cancers of mobile cells such as blood cells disperse throughout the body. Cancer is most often caused by a mutation in the coding or regulatory sequence of a gene whose normal function is to regulate cell division. Such genes are called proto-
There are two basic ways in which translocations can alter the function of proto-
650
The other mechanism by which translocations can cause cancer is the formation of a hybrid gene. An example is provided by the disease chronic myelogenous leukemia (CML), a cancer of white blood cells. This cancer can result from the formation of a hybrid gene between the two proto-
Identifying chromosome mutations by genomics
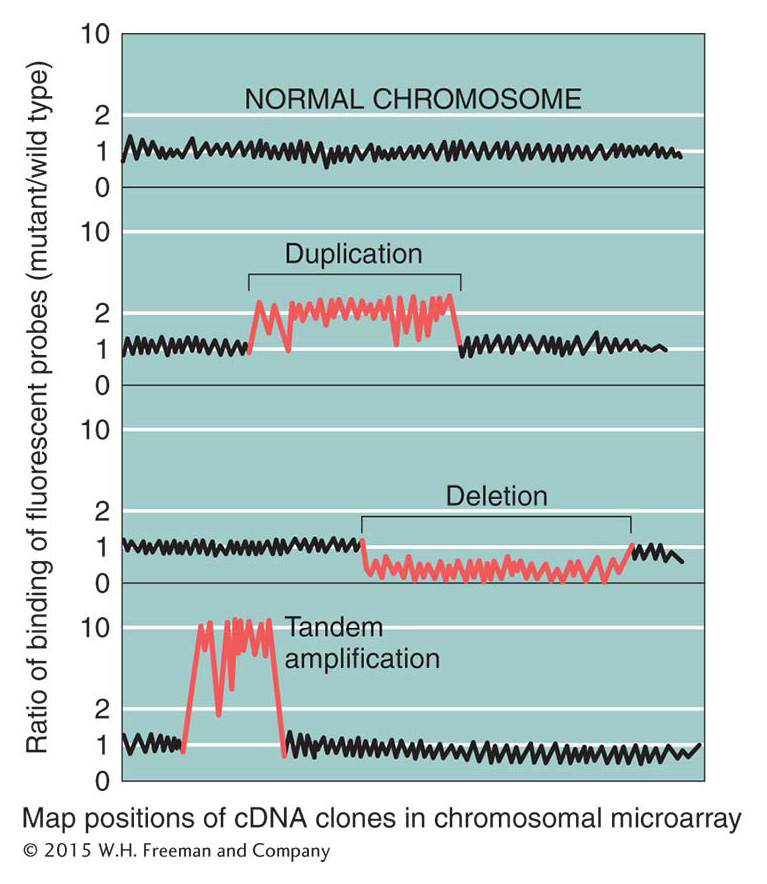
DNA microarrays (see Figure 14-
651