Chapter
3. The let-7 microRNA family: Big roles in development and disease
Introduction
The let-7 microRNA family: Big roles in development and disease
Holly Lewis and Aurora Esquela-Kerscher, Ph.D.
Before the discovery of the first microRNA (miRNA) nearly twenty years ago, RNA was viewed simply as an intermediate messenger in the cell that passes information from nuclear DNA to the cytoplasm, resulting in the translation of messenger RNA (mRNA) into protein. What are miRNAs, and how did they revolutionize our view of RNA function? MiRNAs are small, approximately 22-nucleotide-long, noncoding RNAs that negatively regulate gene expression. They bind to complementary sequences typically located in the 3′ untranslated region (3′ UTR) of their target messenger RNAs (mRNAs) leading to a block in protein translation or mRNA degradation (discussed in Sections 8.5 and 12.7 of your textbook). Although miRNAs were first discovered in worms, it is now clear that thousands of miRNAs exist in the genomes of plants, animals, and viruses. These small RNAs do not encode proteins and yet play major roles in virtually every cellular process, including cellular growth and differentiation, metabolism, immune system surveillance, and aging. The let-7 family is one of the most well characterized miRNA families in animal systems, and studies of its members have generated considerable interest in the clinical arena due to their ability to suppress tumor formation. Here we'll explore the initial discovery of miRNAs in the round worm Caenorhabditis elegans (C. elegans). We'll also investigate the unexpected finding that let-7 is a member of a conserved family of miRNAs that play key roles in development and cancer. Finally, we'll consider the promise of let-7 as a cancer therapeutic.
Making a miRNA Is Not Simple
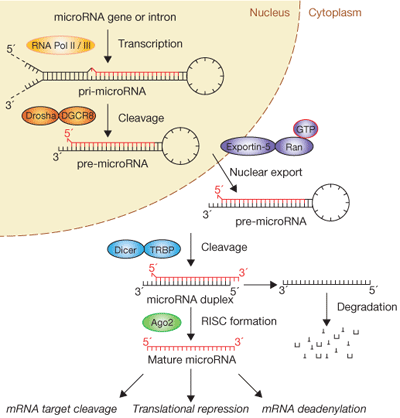
Figure 1: The biogenesis of miRNAs is complex.
Figure 1 Source: Figure 1 from Winter, J. et al. Many roads to maturity: MicroRNA biogenesis pathways and their regulation. Nature Cell Biology 11, 228-234 (2009). doi: 10.1038/ncb0309-228 http://www.nature.com/ncb/journal/v11/n3/fig_tab/ncb0309-228_F1.html
Where are miRNAs located, and how are they produced in cells? miRNA genes can exist individually or in clusters within interagency regions on chromosomes far from other genes, within exons of noncoding genes, or within gene introns. As described in your textbook (Sections 8.5 and 12.7), the generation of a mature, approximately 22-nucleotide-long miRNA is a multistep process in which a large miRNA precursor transcript gets progressively modified and cleaved in both the nuclear and cytoplasmic compartments of the cell1, 2. The biogenesis of miRNAs is extremely complex (Figure 1), and the cell's necessity to undergo multiple processing steps before generating a biologically active “mature” miRNA likely speaks to the functional importance of these small RNAs. A mature miRNA can negatively regulate tens to hundreds of different mRNA targets, and a single mRNA target can be controlled by multiple miRNAs, reflecting the intricate cellular network shared between protein-coding and noncoding RNAs.
1.Bartel, D. P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 116, 281-297 (2004). doi: 10.1016/S0092-8674(04)00045-5
2. Filipowicz, W., Bhattacharyya, S. N. & Sonenberg, N. Mechanisms of post-transcriptional regulation by microRNAs: Are the answers in sight? Nature Reviews Genetics 9, 102-114 (2008). doi: 10.1038/nrg2290
In the Beginning There Were Only Two
During the lifecycle of C. elegans, wild-type worms pass through four distinct larval stages (called L1-L4) before generating a fertile adult worm. The time the nematode spends in each larval stage is invariant from worm to worm, and this developmental timing is regulated by factors belonging to the “heterochronic pathway.” In studies of worms carrying the lineage-defective 4 (lin-4) mutation, Victor Ambros and colleagues discovered that certain groups of cells in these mutant worms fail to progress past the larval 1 (L1) stage of development. After carrying out experiments to map the chromosomal location of the lin-4 gene, they were puzzled to discover that the gene resided in a region of the genome that was devoid of protein-coding genes. How could a mutation in a noncoding region of the genome result in such striking morphological abnormalities in this simple, approximately 960-cell worm? In subsequent experiments, they determined that lin--4 encodes a tiny, approximately 22-nucleotide-long RNA transcript that functions as a “developmental switch” to control the timing of cell differentiation in worms. In 1993, they surmised that the noncoding lin-4 RNA promoted the L1-to-L2 larval transition in worms by physically binding with imperfect complementarity to target sequences located within the 3′ UTR of the lin-14 mRNA, which specifically blocked the expression of the lin-14-encoded protein3. Although these experiments identified lin-4 as the first miRNA, the effect of this discovery was not realized until nearly a decade later.
Seven years later, in 2000, Gary Ruvkun's group identified another noncoding RNA of similar size in C. elegans, called lethal-7 (let-7), which also acted as a timing switch during worm development4. This group found that let-7 was required later during the worm life cycle to control the transition from the larval L4 stage to the adult stage. Similar to the finding that lin-4 regulates the expression of the lin-14 target gene, these scientists discovered that let-7 binds to the 3′ UTR of its target genes, lin-41 and hbl-1, and suppresses the expression of their encoded proteins4, 5. Mutations in the let-7 gene lead to uncontrolled cellular division and a loss of differentiation in a specialized group of cells in the worm called hypodermal seam cells. Notably, uncontrolled cell division and loss of cell differentiation are also hallmarks of cancerous cells and highlight the value of using C. elegans as a simple animal model to understand human disease. Worms carrying mutations in let-7 also have severe (and lethal) morphological defects in the egg-laying structure (the vulva). Collectively, these findings provided evidence that let-7 played multiple roles during nematode development.
3.Lee, R. C., Feinbaum, R. L. & Ambros, V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 75, 843-854 (1993). doi: 10.1016/0092-8674(93)90529-Y
4.Reinhart, B. et al. The 21 nucleotide let-7 RNA regulates C. elegans developmental timing. Nature 403, 901-906 (2000). doi: 10.1038/35002607
5.Slack, F. J. et al. The lin-41 RBCC gene acts in the C. elegans heterochronic pathway between the let-7 regulatory RNA and the lin-29 transcription factor. Molecular Cell 5, 659-669 (2000). doi: 10.1016/S1097-2765(00)80245-2
A Novel RNA Field Is Born
Little attention was paid to the discovery of lin-4 and let-7 outside of the C. elegans community because researchers assumed that this “freak-of-nature” noncoding RNA mechanism controlling developmental timing was unique to round worms. This perception quickly changed when Ruvkun's laboratory reported a few months later that let-7 was 100% conserved across animal phyla, from worms to flies to humans (Figure 2), as well as in marine invertebrates such as coral and mollusks6. Furthermore, the expression of mature let-7 occurs during later stages of development across species, suggesting that temporal control by a miRNA is a universal mechanism. From this “eureka” moment a new field was born.
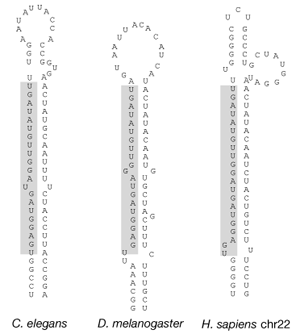
Figure 2: The let-7 miRNA is evolutionarily conserved.
Figure 1 (panel a only) from Pasquinelli, A. E. et al. Conservation of the sequence and temporal expression of let-7 heterochronic regulatory RNA. Nature 408, 86-89 (2000). doi: 10.1038/35040556, http://www.nature.com/nature/journal/v408/n6808/fig_tab/408086a0_F1.html
Less than a year after these initial papers describing let-7 were published, a flurry of work emerged from the Ambros, Bartel, and Tuschel labs that reported the existence of additional, approximately 22-nucleotide-long miRNAs in invertebrate and vertebrate genomes using both bioinformatic approaches and direct small RNA cloning techniques7, 8, 9. Soon it was clear that thousands upon thousands of miRNA genes existed in virtually every organism studied, including the single-cell green algae Chlamydomonas reinhardtii10. The human genome alone encodes more than 1,400 distinct miRNA genes, which account for approximately 3% of all expressed human genes, establishing miRNAs as one of the largest classes of gene regulators11. Notable exceptions do exist, however, and miRNAs are absent in fungi and some unicellular species, including Tetrahymena thermophila. Following in the footsteps of the let-7 family, it is estimated that one third of all miRNAs are highly conserved across species and likely control essential and ancient functions in animal systems.
6.Pasquinelli, A. et al. Conservation of the sequence and temporal expression of let-7 heterochronic regulatory RNA. Nature 408, 86-89 (2000). doi: 10.1038/35040556
7.Lau, N. C. et al. An abundant class of tiny RNAs with probable regulatory roles in Caenorhabditis elegans. Science 294, 858-862 (2001). doi: 10.1126/science.1065062
8.Lagos-Quintana, M. et al. Identification of novel genes coding for small expressed RNAs. Science 294, 853-858 (2001). doi: 10.1126/science.1064921
9.Lee, R. C. & Ambros, V. An extensive class of small RNAs in Caenorhabditis elegans. Science 294, 862-864 (2001). doi: 10.1126/science.1065329
10.Zhao, T. et al. A complex system of small RNAs in the unicellular green alga Chlamydomonas reinhardtii. Genes and Development 21, 1190-1203 (2007). doi: 10.1101/gad.1543507
11.Griffiths-Jones, S. H., van Dongen, S. & Enright, A. J. miRBase: Tools for microRNA genomics. Wellcome Trust, Sanger Institute (https://www.sanger.ac.uk/) (2008). doi: 10.1093/nar/gkm952
The Role of the let-7 Family during Development
Due to the highly conserved nature of let-7 across species, scientists have focused much attention on this miRNA family to understand the functional significance of miRNAs during development and disease. What are some of the developmental roles of let-7 family members? Are the functions of let-7 conserved across species? The let-7 miRNA family includes eight closely related homologues in the C. elegans genome: miR-48, miR-84, miR-241, miR-265, miR-793, miR-794, miR-795, and miR-1821. Although the functions of miR-265, miR-793, miR-794, miR-795, and miR-1821 have not been studied, the other three let-7 homologues (miR-48, miR-84, and miR-241) exhibit distinct spatial and temporal expression patterns during the C. elegans life cycle12, 13, 14. Similar to let-7, these miRNAs participate in the heterochronic pathway and act as “developmental switches.” Notably, miR-48, miR-84, and miR-241 function in a redundant manner to control the L2-to-L3 larval transition in worms15, whereas let-7 is required later during C. elegans development to direct the L4-to-adult transition.
In mammals, the let-7 family is even larger than in C. elegans, consisting of thirteen closely related family members. Not much is known about the developmental role of these miRNAs in mammals, likely because the mammalian let-7 homologues carry out overlapping functions; thus, multiple family members would need to be disrupted in the same animal so as to study their biological effects. What about the expression patterns of mammalian let-7 family members? Studies in mice show that let-7 members are expressed during mid- to late-stage embryogenesis, but they are not expressed during the earliest developmental stages or in embryonic stem cells. These findings suggest that the let-7 family members possess a conserved role in promoting cellular differentiation and cell division cycle control16, 17, 18, 19, 20, 21. Additional data indicate that let-7 homologues play important roles in limb and brain development as well as during neuronal differentiation in mammals19, 20, 21, 22, 23, 24, 25.
12. Li, M. et al. Regulatory mutations of mir-48, a C. elegans let-7 family MicroRNA, cause developmental timing defects. Developmental Cell 9, 415-422 (2005). doi: 10.1016/j.devcel.2005.08.002
13.Johnson, S. M. et al. RAS is regulated by the let-7 microRNA family. Cell 120, 635-647 (2005). doi: 10.1016/j.cell.2005.01.014
14.Esquela-Kerscher, A. et al. Post-embryonic expression of C. elegans microRNAs belonging to the lin-4 and let-7 families in the hypodermis and the reproductive system. Developmental Dynamics 234, 868-877 (2005). doi: 10.1002/dvdy.20572
15.Abbott, A. L. et al. The let-7 microRNA family members mir-48, mir-84, and mir-241 function together to regulate developmental timing in Caenorhabditis elegans. Developmental Cell 9, 403-414 (2005). doi: 10.1016/j.devcel.2005.07.009
16.Thomson, J. M. et al. Extensive post-transcriptional regulation of microRNAs and its implications for cancer. Genes and Development 20, 2202-2207 (2006). doi: 10.1101/gad.1444406
17.Wienholds, E. & Plasterk, R. H. MicroRNA function in animal development. FEBS Letters 579, 5911-5922 (2005). doi: 10.1016/j.febslet.2005.07.070
18.Wulczyn, F. G. et al. Post-transcriptional regulation of the let-7 microRNA during neural cell specification. FASEB Journal 21, 415-426 (2007). doi: 10.1096/fj.06-6130com
19.Rybak, A. et al. A feedback loop comprising lin-28 and let-7 controls pre-let-7 maturation during neural stem-cell commitment. Nature Cell Biology 10, 987-993 (2008). doi: 10.1038/ncb1759
20.Schulman, B. R., Esquela-Kerscher, A. & Slack, F. J. Reciprocal expression of lin-41 and the microRNAs let-7 and mir-125 during mouse embryogenesis. Developmental Dynamics 234, 1046-1054 (2005). doi: 10.1002/dvdy.20599
21.Lee, Y. S. et al. Depletion of human micro-RNA miR-125b reveals that it is critical for the proliferation of differentiated cells but not for the down-regulation of putative targets during differentiation. Journal of Biological Chemistry 280, 16635-16641 (2005). doi: 10.1074/jbc.M412247200
22.Mansfield, J. H. et al. MicroRNA-responsive "sensor" transgenes uncover Hox-like and other developmentally regulated patterns of vertebrate microRNA expression. Nature Genetics 36, 1079-1083 (2004). doi: 10.1038/ng1421
23.Lagos-Quintana, M. et al. Identification of tissue-specific microRNAs from mouse. Current Biology 12, 735-739 (2002). doi: 10.1016/S0960-9822(02)00809-6
24.Sempere, L. F. et al. Expression profiling of mammalian microRNAs uncovers a subset of brain-expressed microRNAs with possible roles in murine and human neuronal differentiation. Genome Biology 5, R13 (2004).
25.Wu, L. & Belasco, J. G. Micro-RNA regulation of the mammalian lin-28 gene during neuronal differentiation of embryonal carcinoma cells. Molecular Cell Biology 25, 9198-9208 (2005). doi: 10.1128/MCB.25.21.9198-9208.2005
The Link between let-7 and Cancer
In 2004, Carlo Croce and colleagues were the first to make a direct connection between the let-7 miRNA family and human cancer26. This breakthrough happened when they mapped the location of 186 miRNA genes to human chromosomes and realized that almost half of them resided within unstable areas of the genome that are prone to deletions, amplifications, viral insertions, and chromosome fusions. These “fragile” chromosomal areas are commonly associated with human disease. Many let-7 homologues mapped within fragile sites that are often deleted in lung, breast, ovarian, cervical, and urothelial cancers26. In particular, the let-7g miRNA maps to a specific region of human chromosome 3 (called 3p21) that is often deleted during the early stages of lung cancer. Therefore, the let-7 family members are predicted to act as tumor suppressor genes, factors that curb the rate of cellular growth and division and are often mutated in cancerous cells. When tumor suppressor genes are mutated or deleted, the resulting cells often grow and divide in an uncontrollable manner and eventually become cancerous.
If loss of let-7 miRNA function or expression leads to cancer, would increased expression of let-7 miRNAs prevent cells from becoming cancerous? Junichi Takamizawa and colleagues provided experimental support for this hypothesis and showed that artificial overexpression of let-7 in human cancer cells decreased their rates of cell division. They also suggested that let-7 expression levels could be used to predict disease outcomes in cancer patients. They found that lung cancer patients with lower levels of let-7 expression in their tumors exhibited a worse survival rate after the tumor was surgically removed than lung cancer patients whose tumors had higher levels of let-7 expression27. Although evidence was accumulating that let-7 might indeed function to inhibit cancer, no cancer-related let-7 target genes had been identified to substantiate these claims.
How did researchers identify a more direct connection between let-7 and cancer? Frank Slack's laboratory turned back to the powerful C. elegans genetic model to try to gain a better understanding how let-7 regulates cell division and differentiation pathways in animals. Researchers used bioinformatic approaches to search for clues about what types of mRNA targets may be regulated by let-7, and they screened the 3′ UTRs of all known protein-coding genes in C. elegans for let-7 binding sites. One of the top hits was the let-60 gene, which is most closely related to the RAS oncogenes in humans. RAS is a membrane-associated GTPase signaling protein that promotes cell growth and proliferation. Mutations that lead to increased RAS activity are commonly found in human cancers. Slack and colleagues confirmed that let-7 can bind to the 3′ UTR of let-60/RAS in C. elegans and down-regulate its expression.
The worm data looked great, but one big question remained: does let-7 also function to suppress RAS expression in humans? Indeed, the 3′ UTRs of all three human RAS orthologs—HRAS, KRAS, and NRAS—contain multiple binding sites for let-7. More compelling evidence showed that the let-7 family members could directly inhibit RAS protein expression in experiments using cultured human cancer cells13. What about human patients with cancer? If let-7 does indeed function as a tumor suppressor that keeps the levels of the RAS oncogene in check, then one would hypothesize that let-7 expression levels would be unusually low and that RAS protein levels would be unusually high in tumor cells compared with healthy cells. To address this question, the Slack lab compared let-7 and RAS expression levels in lung tumor tissue and adjacent normal lung tissue from lung cancer patients. As predicted, they found that let-7 levels were down-regulated and that RAS protein levels were up-regulated in the lung tumor tissue13; in contrast, the opposite was true in adjacent normal lung tissue from the same patients.
Following this pivotal study, work in the Slack lab as well as findings from other laboratories determined that let-7, likely functions as a “master regulator” of cellular growth and division because the let-7 family in mammals regulates the expression of additional cancer-related genes, including MYC, HMGA2, Cyclin D, CDK6, and CDC25A, all of which promote cellular growth28, 29, 30, 31, 32. Since the link between let-7 expression and lung cancer was established, scientists have gone on to measure the expression levels of let-7 family members in many other types of cancers and found that they are often lower in cancerous tissues relative to normal tissues33, 34. Furthermore, the overexpression of members of the let-7 family can inhibit the growth and division of a wide range of human cancer cell lines, including breast, cervical, and liver cancer cell lines28, 35, 36, 37. These studies indicate that members of the let-7 family are likely to act as essential tumor suppressor genes in humans.
26.Calin, G. A. et al. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. PNAS 101, 2999-3004 (2004). doi: 10.1073/pnas.0307323101
27.Takamizawa, J. et al. Reduced expression of the let-7 microRNAs in human lung cancers in association with shortened postoperative survival. Cancer Research 64, 3753-3756 (2004). doi: 10.1158/0008-5472.CAN-04-0637
28.Johnson, C. D. et al. The let-7 microRNA represses cell proliferation pathways in human cells. ancer Research 67, 7713-7722 (2007). doi: 10.1158/0008-5472.CAN-07-1083
29.Grosshans, H. et al. The temporal patterning microRNA let-7 regulates several transcription factors at the larval to adult transition in C. elegans. Developmental Cell 8, 321-330 (2005). doi: 10.1016/j.devcel.2004.12.019
30.Lee, Y. S. & Dutta, A. The tumor suppressor microRNA let-7 represses the HMGA2 oncogene. Genes Development 21, 1025-1030 (2007). doi: 10.1101/gad.1540407
31.Mayr, C., Hemann, M. T. & Bartel, D. P. Disrupting the pairing between let-7 and Hmga2 enhances oncogenic transformation. Science 315, 1576-1579 (2007). doi: 10.1126/science.1137999
32.Sampson, V. B. et al. MicroRNA let-7a down-regulates MYC and reverts MYC-induced growth in Burkitt lymphoma cells. Cancer Research 67, 9762-9770 (2007). doi: 10.1158/0008-5472.CAN-07-2462
The Promise of let-7 as a Therapeutic for Cancer
If let-7 could block cancer cell growth in in vitro experiments using cultured human cancer cells, could this tiny RNA block tumor growth in animals that are prone to cancer? Studies in the Slack laboratory and at Asuragen quickly tested this possibility. First, they found that tumor formation in mice injected with human lung cancer cells could be dramatically blocked if these cells were first treated with let-7 before they were injected into mice when compared with animals injected with untreated human cancer cells36. Although the results of these experiments provided compelling evidence that let-7 can inhibit tumor formation, these studies were still far removed from what occurs in an animal that develops tumors from its own cells.
To convincingly show that let-7 functions as a bona fide tumor suppressor gene in the lung, they turned to a special strain of mice genetically engineered to express a mutant form of RAS (called KRAS G12D) in their lungs. This particular mutation locks RAS into its active state and causes it to continuously signal lung cells to grow and divide. All the mice that express this mutant form of RAS in their lungs develop lung cancer.38 If let-7 functions as a tumor suppressor by inhibiting RAS expression, would high levels of let-7 inhibit tumor formation in the KRAS G12D mice? Indeed, when let-7 was administered to the lungs of these mice, they discovered that let-7 treatment resulted in a dramatic reduction in lung tumor formation36. As a proof-of-principle experiment, this work convincingly showed that let-7 could suppress tumor growth in living animals. Similar results were also reported by colleagues in Tyler Jack's laboratory35. Subsequent studies by Slack's group showed that treatment with let-7 miRNA could also shrink preexisting lung tumors in KRAS G12D mice39. These results indicate that let-7 could be useful in the clinic for diagnosing and treating cancer.
Summary
The let-7 miRNA family holds great promise as a therapy to treat lung cancer in humans. These miRNAs could potentially be effective in treating a wide array of cancers outside of the lung because they regulate cell growth and division at multiple levels. Studies in C. elegans have provided invaluable insights into the role of let-7 family members in our own bodies. With the ongoing identification of novel miRNA genes, more surprises and groundbreaking discoveries are sure to follow in this growing and exciting field.
References
3.Lee, R. C., Feinbaum, R. L. & Ambros, V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 75, 843-854 (1993). doi: 10.1016/0092-8674(93)90529-Y
4.Reinhart, B. et al. The 21 nucleotide let-7 RNA regulates C. elegans developmental timing. Nature 403, 901-906 (2000). doi: 10.1038/35002607
5.Slack, F. J. et al. The lin-41 RBCC gene acts in the C. elegans heterochronic pathway between the let-7 regulatory RNA and the lin-29 transcription factor. Molecular Cell 5, 659-669 (2000). doi: 10.1016/S1097-2765(00)80245-2
6.Pasquinelli, A. et al. Conservation of the sequence and temporal expression of let-7 heterochronic regulatory RNA. Nature 408, 86-89 (2000). doi: 10.1038/35040556
7.Lau, N. C. et al. An abundant class of tiny RNAs with probable regulatory roles in Caenorhabditis elegans. Science 294, 858-862 (2001). doi: 10.1126/science.1065062
8.Lagos-Quintana, M. et al. Identification of novel genes coding for small expressed RNAs. Science 294, 853-858 (2001). doi: 10.1126/science.1064921
9.Lee, R. C. & Ambros, V. An extensive class of small RNAs in Caenorhabditis elegans. Science 294, 862-864 (2001). doi: 10.1126/science.1065329
10.Zhao, T. et al. A complex system of small RNAs in the unicellular green alga Chlamydomonas reinhardtii. Genes and Development 21, 1190-1203 (2007). doi: 10.1101/gad.1543507
11.Griffiths-Jones, S. H., van Dongen, S. & Enright, A. J. miRBase: Tools for microRNA genomics. Wellcome Trust, Sanger Institute (https://www.sanger.ac.uk/) (2008). doi: 10.1093/nar/gkm952
12. Li, M. et al. Regulatory mutations of mir-48, a C. elegans let-7 family MicroRNA, cause developmental timing defects. Developmental Cell 9, 415-422 (2005). doi: 10.1016/j.devcel.2005.08.002
13.Johnson, S. M. et al. RAS is regulated by the let-7 microRNA family. Cell 120, 635-647 (2005). doi: 10.1016/j.cell.2005.01.014
14.Esquela-Kerscher, A. et al. Post-embryonic expression of C. elegans microRNAs belonging to the lin-4 and let-7 families in the hypodermis and the reproductive system. Developmental Dynamics 234, 868-877 (2005). doi: 10.1002/dvdy.20572
15.Abbott, A. L. et al. The let-7 microRNA family members mir-48, mir-84, and mir-241 function together to regulate developmental timing in Caenorhabditis elegans. Developmental Cell 9, 403-414 (2005). doi: 10.1016/j.devcel.2005.07.009
16.Thomson, J. M. et al. Extensive post-transcriptional regulation of microRNAs and its implications for cancer. Genes and Development 20, 2202-2207 (2006). doi: 10.1101/gad.1444406
17.Wienholds, E. & Plasterk, R. H. MicroRNA function in animal development. FEBS Letters 579, 5911-5922 (2005). doi: 10.1016/j.febslet.2005.07.070
18.Wulczyn, F. G. et al. Post-transcriptional regulation of the let-7 microRNA during neural cell specification. FASEB Journal 21, 415-426 (2007). doi: 10.1096/fj.06-6130com
19.Rybak, A. et al. A feedback loop comprising lin-28 and let-7 controls pre-let-7 maturation during neural stem-cell commitment. Nature Cell Biology 10, 987-993 (2008). doi: 10.1038/ncb1759
20.Schulman, B. R., Esquela-Kerscher, A. & Slack, F. J. Reciprocal expression of lin-41 and the microRNAs let-7 and mir-125 during mouse embryogenesis. Developmental Dynamics 234, 1046-1054 (2005). doi: 10.1002/dvdy.20599
21.Lee, Y. S. et al. Depletion of human micro-RNA miR-125b reveals that it is critical for the proliferation of differentiated cells but not for the down-regulation of putative targets during differentiation. Journal of Biological Chemistry 280, 16635-16641 (2005). doi: 10.1074/jbc.M412247200
22.Mansfield, J. H. et al. MicroRNA-responsive "sensor" transgenes uncover Hox-like and other developmentally regulated patterns of vertebrate microRNA expression. Nature Genetics 36, 1079-1083 (2004). doi: 10.1038/ng1421
23.Lagos-Quintana, M. et al. Identification of tissue-specific microRNAs from mouse. Current Biology 12, 735-739 (2002). doi: 10.1016/S0960-9822(02)00809-6
24.Sempere, L. F. et al. Expression profiling of mammalian microRNAs uncovers a subset of brain-expressed microRNAs with possible roles in murine and human neuronal differentiation. Genome Biology 5, R13 (2004).
25.Wu, L. & Belasco, J. G. Micro-RNA regulation of the mammalian lin-28 gene during neuronal differentiation of embryonal carcinoma cells. Molecular Cell Biology 25, 9198-9208 (2005). doi: 10.1128/MCB.25.21.9198-9208.2005
26.Calin, G. A. et al. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. PNAS 101, 2999-3004 (2004). doi: 10.1073/pnas.0307323101
27.Takamizawa, J. et al. Reduced expression of the let-7 microRNAs in human lung cancers in association with shortened postoperative survival. Cancer Research 64, 3753-3756 (2004). doi: 10.1158/0008-5472.CAN-04-0637
28.Johnson, C. D. et al. The let-7 microRNA represses cell proliferation pathways in human cells. ancer Research 67, 7713-7722 (2007). doi: 10.1158/0008-5472.CAN-07-1083
29.Grosshans, H. et al. The temporal patterning microRNA let-7 regulates several transcription factors at the larval to adult transition in C. elegans. Developmental Cell 8, 321-330 (2005). doi: 10.1016/j.devcel.2004.12.019
30.Lee, Y. S. & Dutta, A. The tumor suppressor microRNA let-7 represses the HMGA2 oncogene. Genes Development 21, 1025-1030 (2007). doi: 10.1101/gad.1540407
31.Mayr, C., Hemann, M. T. & Bartel, D. P. Disrupting the pairing between let-7 and Hmga2 enhances oncogenic transformation. Science 315, 1576-1579 (2007). doi: 10.1126/science.1137999
32.Sampson, V. B. et al. MicroRNA let-7a down-regulates MYC and reverts MYC-induced growth in Burkitt lymphoma cells. Cancer Research 67, 9762-9770 (2007). doi: 10.1158/0008-5472.CAN-07-2462
Quiz
Question
3.1
FrV9IDzso9cq8j0aEfkcAlE38OTx0QKjzdiCcPQl5PXErnyv99t0t2gVHhv0dWjXIsaDceYXuCHf3JXZ1alngYOxiytNgMA6HApzfaP+AdKdHXh1ZHoCEtV5DqtTsN7+otWAoKTufnr/8Ghh+0c01pIs269RIXBKSKrd1zW6dQzHLshCI2Lh0Mx1EUAw597YhLvfef8HyFa1LazxhEpL90OYzqeuthuQmMzfsQbAwrzixE6MjqZgMquKGi2HGAjThDMZMr2NbcLdmyTyo3W/CucG9w6TSzujbi8oV15osl4GxRl6QTKDzKfdP1+pcMimzhAmwxmyn0j3SAoucKnPSZu+3scKE0MnONuXkamOHajvejQaQP1Wuepv5DQ=
1. How does the nature of the let 7 gene's product distinguish it from other tumor suppressor genes?
Question
3.2
iG+ZyXNW9VDm1fN+xOCWQqbiooCjoSOi7JrNPfSHvtEzr/3WYq2ty1Lgx21Rb3rhCBWy4wJ8fxYKdxK4pi2skiLikIZfUftncy8/KbYJD0HEagrqas6mzlhPDsvDNcg/lNBB2xstAHNEEKqAtfutlKQe2j47WKDwMM/+SGajzaz8Hw+03fwoFMaWTws+I/O/kmWdnAxsx4Ls9QrEtZmSLlspVhDRuY624XgP7Uf/KLUHzZtfAbEYrpJs11uR6R/UrCAbhP5sixiunageFd27BZDbpgGk7i2a0z83L69KqCzPyDvbLRhOjmyRWdB1RkNtenNKIomf5r/++dgwYcoGvwtrrbAw2i3D
2. After let 7 is induced in tumors what change would you NOT expect to happen?
Question
3.3
mHqQyspsEQvc0fQ719jqdh5fDUfHjC/m/6ApKuqgsNy/612zrjELJgKNNkq/2SZ4XIyiqUJCGBbCBycrZMlMekzIshiLYgZej6WSfmzjfy1rOvrdMo30HXSBiK4hO20GcoJ6BohxtldCpkeTBSUFmFfvOqhBnLuFTj1ltpqf2bqDP0hYzTCeXZJGf2o0HioBFrfp7cB2XAqB2JPoYhZVKR4HBwNNzZG/98eW3kFxFerBpCC92CysL8hRXPcNutLHoNeku0SgspAW5gEBEp185baB+jfzHuBZlguY4k/EIFx7Lj/zsclXedDkN5bCC0VE+RB6KOis0A3kX4Q9OwcUFNUmGUsiOwI24QZSA+Mq3WQbLVJLxCiWu1u5I1qEsTFVKLHS/EPmP2BZ3YqzUkYCXvnJpLluuC0dxrbi4vC+Jj1aUo9xZtsi5Nk8od4wRAopYQ53vWmW+torDycCnC6tlh9/1nZvPaD/4LqZTuMMpYkoxXeR3LciCJLqkD7bkVFpFnwgpafyMTTFfg4w7uZxQdFyArQruNl/NthfHuHNLOQiCBqIX3awIVTg3xkyp4KSP+m3YbnCFv7PoK5QIIIW7Y7CnNM6tdji
3. let 7 and other miRNA genes are conserved between species. Why is this observation important to researchers?
Question
3.4
uEbAEKh+6ecyiF3VOCQ3E04GvjqgTgRCPWojuD3xRVLbAkK0jBKG06XrBWTQ+eC4fwL2hTZnH4MqABctx9kKMRa3Z4slXprd1JsIlZ4if/vXLWZrNNFciWVDP5r+dyKewZfws6E5QdHrFdmoJLT2T6aKNhfWCLqDdFm39dnh3VsVjPzO45Pk07Uw3lT65vm87Xq7k8V0CUaiqtQGmXfC18hEKsI+FSRtSJN2+xKnYqZSv/6VgcygqQ1Le1Uhb0aT2vOuETMrGToTrIYatkQjISUhSxG387B+mu/sG862pmbWwPhey3yPb+n1L1qDEATqPBS9UyTpzCyryztOiCukqZ+WpUhuNwVoms6YmLbKFO/9a5/b63mlKq9mTgrnKRMQUiHoQ/HLIec=
4. Which of the following is a characteristic of miRNA genes?
Question
3.5
csH7wZqkpuYSk1JJkdfYb0mUv5Cy28h47Xpz7WgL9LR4Dn9DvAmv11OG3P8LFKGbkolfNCsd/hfdP5hhA5VslEWNatPom0fEhNAGeznjcRLG5UTGCF/W1PRiBTsW9B2Pxc49hpgXaAfmCJawFLnVbwT0tg005A3Tzss1d1J0/J3bKRwxdJ+XA5QnLMr377fU31HahuX2nsdesRwh0nei23dWDnWWg5gZM9qQ8YsN10b0LMkCFvkpqj0kZf3OEwNMzN1ARQjSDkooYgsa+O1bkJWjrWSQfrHG1Fn6DuOUWI6PI0HNaC6S3HcQkzntJnknj/KwAg==
5. Which of the following changes could result in decreased levels of the let 7 miRNA?
Question
3.6
Q6NkWBV8MKv9/9VW1cJEMhGvAK4rOSVEOMIrolZlkxUhKbr5wJByLnuNFYSyRDfa1jM7mWsjwLbNZrLRjzj+wYjxkFSd2I9yphvnQGfeAoyRemwoJjDbPunzz1nT11CVlPJzCL7vvM/8pCYpq690GKSlcVjAZS3Ki/QUubhSqLqKe5GWgJCChtkCb2Y0CDrk4RIiW0zHE6bgSi/XMcXVdAlf4FXmZuJi8G2/sd+hhGT6h/Vi3m1/XSjuRmxa+KF+a5zq75dWqMAXQFSdiNOdHF47J/Y3B0ISENnx9IP5SeBaf3Z5jxz0fxTDaxFxF+BQBnN7fJftA7Gw0XqUqdXEwkt2dRQ+p8t/Ybr7mbRlwe3CnhzLQ55iBj0I5hvLx/UC1KJHuZd5ucWqFZH900dlxtJvTWc5mk6dvWm2dN7UBwqHc+M1vnmKPsSI9eGsMdtYTpfT/oaDabTWW5TKeV4UrTzNYj7HsLKGBFYr/JZiw404PPxylJ3zaoYvPoXQ9jGzSGMwl4UPahhljm5Xl7IgmXlb7PwqoDyWW6mGVOlVok93bDqKPYDU9jQ7ZI8Lcn039KpQA7ZUPtYD2h69dKdD2kTvFLE=
6. You found a mutation causing a strong phenotype, but the mutation is located in a non-coding region of the genome. Which of the following statements is NOT a possible explanation of your data?
Question
3.7
gGf6IgMUqHrT5ejBEWKzHU/gDqmpRbL7IjNxgkf3/WhBfAaiI7lQvmCNaZ8x8qrSpDNI0/djsr9JdY9Em3yOIu9Jx3Yc/HywpIReSQknlafp2g4G7P0UVsa1nPzPIMx0V2tsFulQPP48nex1dMS5ObeupcLUyTFlK3fqCrgO8dap9WNRi2PjlXOxwKjvYc/jk8o3J1o8qJVIKwbxsjZzhx5F3UqAeTtol2XD4LwEecZYwp4r4CELPkcOXKVZc9nd6c1VKXD1P/nCf9ePqQgIetcKh9RLAHJecoe4lD8oeUWf8LJOPL2Lc1jSyrmlrz+C7xdCuOCSPoOwDbLI6nKt82JemneXsvtFwMv69rp5GK3vmbBwtw9V276kzOI48scfY73UMsyn5y0zINYj1GB9JRaifhHGMa5GCSwSxhhcNBxuXoqCJI0qB7nPSpsV8pAPGks7bLrqzWybd72vQ+xod/JCfGoHk1/fkodGzSU83mOxlClV/z1Pl9bxXvIujRCFsYzmQwqHetnttHkgLieYO71mYSkRZ2wVKr5CMDiqTmo=
7. Your patient's tumor has very high levels of the oncogene MYC. Which of these statements is a possible explanation for misregulated MYC?

