11-3 Basal Ganglia, Cerebellum, and Movement
The main evidence that the basal ganglia and the cerebellum perform motor functions is that damage to either structure impairs movement. Both have extensive connections with the motor cortex, which further suggests their participation in movement. After an overview of each structure’s anatomy, we look at some symptoms that arise after damage to the basal ganglia or the cerebellum. Then we consider the roles each structure plays in controlling movement.
Basal Ganglia and the Force of Movement
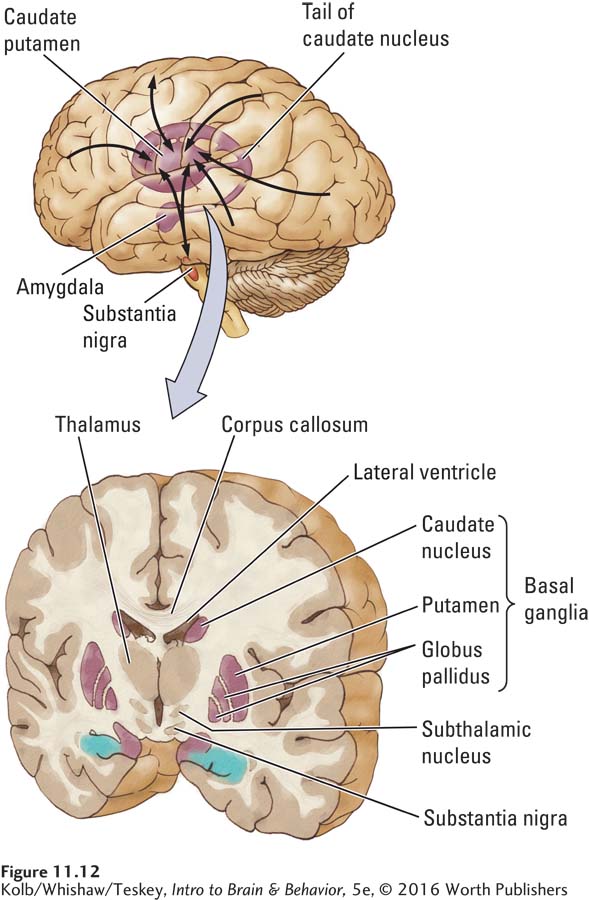
The neocortex connects extensively with the basal ganglia, a collection of nuclei lying just beneath the cortex, as illustrated in Figure 11-12. The basal ganglia do not produce movement directly, but rather modulate the activity of cortical motor systems. The nuclei participate in a wide range of functions, including association or habit learning, motivation, and emotion, as well as motor control.
Anatomy of the Basal Ganglia
Among the nuclei forming the basal ganglia are the caudate nucleus and the putamen, which together form the striatum (meaning striped body and named for the fibers, including corticospinal fibers, running through it), and the subthalamic nucleus and globus pallidus. As shown in Figure 11-12, the prominent striatum extends as a tail (caudate means having a tail) into the temporal lobe, ending in the amygdala. There are three main basal ganglia connections:
- All areas of the neocortex and more primitive allocortex project to the basal ganglia.
- The basal ganglia project to the motor cortex via relays in the thalamus.
- The basal ganglia receive connections from the dopamine cells of the midbrain substantia nigra over the nigrostriatal pathway and also project to the substantia nigra.
Figure 5-17 traces the nigrostriatal pathways in the dopaminergic activating system and highlights their importance for maintaining healthy motor behavior.
Through these connections, the basal ganglia form reciprocal circuits, or loops, connecting all neocortical and allocortical regions to the motor cortex. Other loops connect the basal ganglia and substantia nigra, allowing the substantia nigra to modulate the subcortical–
How the Basal Ganglia Control Movement Force
Focus 3-4 describes the genetic basis of Huntington disease.
Two types of movement disorders—
Detailed coverage of Parkinson disease and its treatment appears in Chapters 5, 7, and 16.
374
In addition to causing involuntary movements, or hyperkinetic symptoms, as just described, basal ganglia damage can result in a loss of motor ability, or hypokinetic symptoms that feature rigidity and difficulty initiating and producing movements. Parkinson disease is marked by hypokinetic symptoms caused by the loss of dopamine cells in the substantia nigra that project into the basal ganglia.
The fact that both hyperkinetic and hypokinetic symptoms arise subsequent to basal ganglia damage suggests that one function of these nuclei is regulating movement force. The idea is that hyperkinetic disorders such as Huntington disease result from errors of too much force and so result in excessive movement. Hypokinetic disorders such as Parkinson disease result from errors of too little force and so result in insufficient movement.
In support of these ideas, in a reaching task, Huntington subjects reached using too much force, thus seemingly flinging a limb. Parkinson subject reached with too little force, thus producing slowed movement (Moisello et al., 2011). An MRI study of basal ganglia activity in healthy participants who, for a small monetary reward, considered how much force to apply in a gripping task, showed more basal ganglia activity when they contemplated using a more forceful grip and less activity when contemplating a less forceful grip (Kurniawan et al., 2010). Together, these studies suggest that the basal ganglia play a role not just in producing force but also in computing the effortful costs of making movements.
CLINICAL FOCUS 11-4
Tourette Syndrome
The neurological disorder Tourette syndrome (TS) was first described in 1885 by Georges Gilles de la Tourette, a French neurologist, who described the symptoms as they appeared in Madame de D., one of his patients:
Madame de D., presently age 26, at the age of 7 was afflicted by convulsive movements of the hands and arms. These abnormal movements occurred above all when the child tried to write, causing her to crudely reproduce the letters she was trying to trace. After each spasm, the movements of the hand became more regular and better controlled until another convulsive movement would again interrupt her work. She was felt to be suffering from over-
The statistical incidence of Tourette syndrome is about 1 in 1000 people. TS affects all racial groups and seems to be hereditary. The age range of onset is 2 to 25 years.
The most frequent symptoms of Tourette syndrome are involuntary tics and complex movements, such as hitting, lunging, or jumping. People with TS may also emit sudden cries and other vocalizations or inexplicably utter words that do not make sense in the context, including coprolalia and swearing.
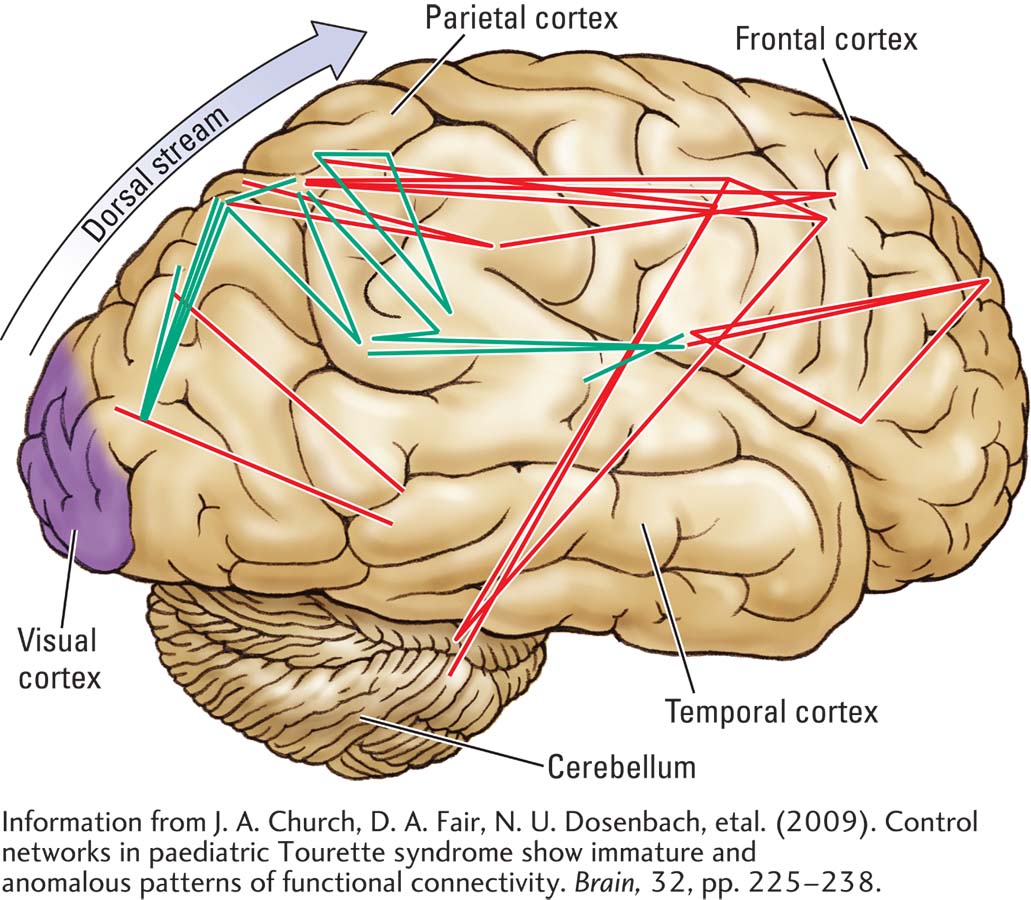
Tourette syndrome is thought to reflect an abnormality of the basal ganglia, especially in the right hemisphere, because its symptoms can be controlled with haloperidol, an antipsychotic drug that blocks dopamine synapses in the basal ganglia. Using fMRI to correlate resting activity with brain regions, Church and colleagues (2009) documented cortical changes associated with TS. These include increased connectivity, mainly in the parietal cortex of Tourette patients, and decreased connectivity between parietal and frontal cortex. This finding, diagrammed in the illustration, suggests altered functioning in neural circuits that connect the posterior sensory regions of the cortex to its anterior motor regions.
The urge to make involuntary movements and vocalizations may be similar to behaviors that have an urge-
375
What features of the reciprocal basal ganglia loops allow for selecting movements or modulating movement force? One theory holds that the basal ganglia can influence whether movement occurs (Friend & Kravitz, 2014). As illustrated in Figure 11-13, a pathway (green) from the thalamus to the cortex to the spinal cord produces movement. The globus pallidus (red) can inhibit this pathway at the level of the thalamus.
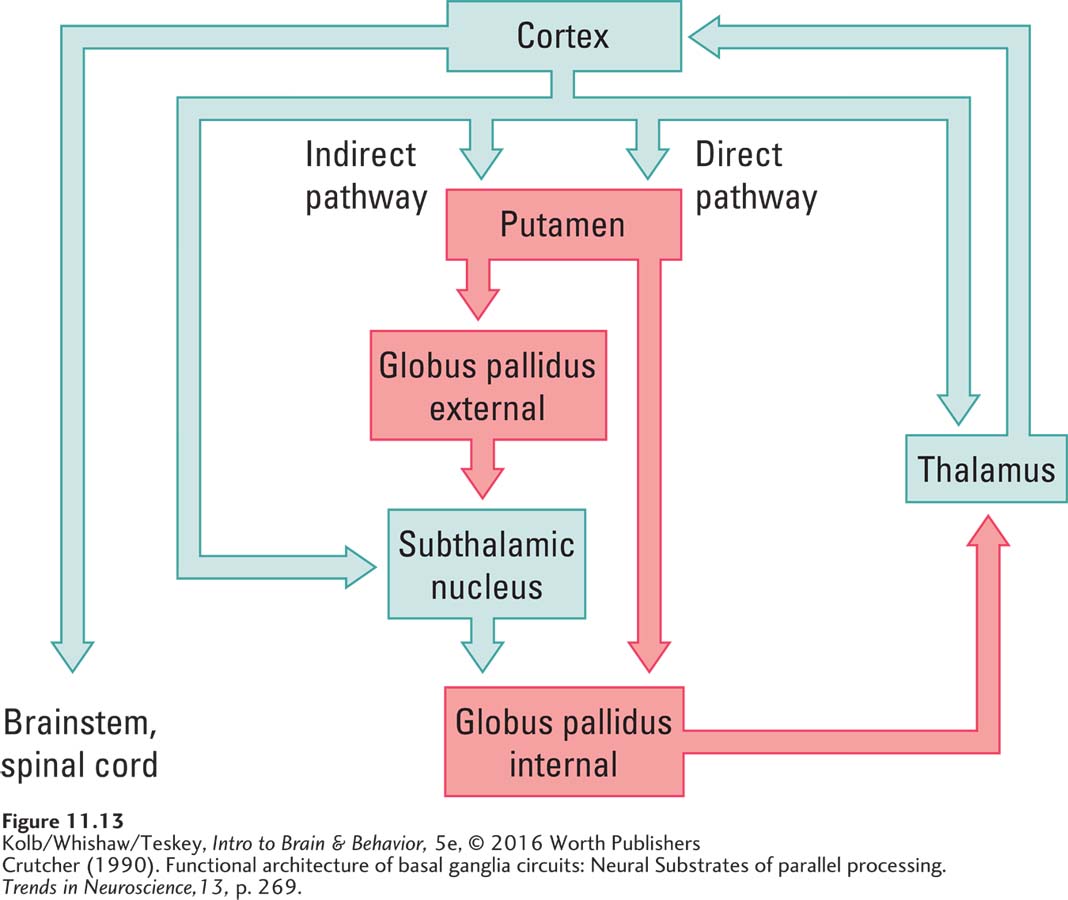
The globus pallidus is controlled by two basal ganglia pathways, one indirect and one direct. If the globus pallidus is excited, it in turn inhibits the thalamus and blocks movement. If it is inhibited, motor cortex circuits that include the thalamus are able to produce movement. The globus pallidus thus acts like a volume control. If it is turned down, movement can occur; if it is turned up, movement is blocked. This model proposes that diseases of the basal ganglia affecting its “volume control” function impair movement so that it is either excessive or slowed.
The idea that the globus pallidus acts like a volume control is the basis for several treatments for Parkinson disease. Consistent with the volume hypothesis, recordings made from globus pallidus cells show excessive activity, which inhibits movement in people with Parkinson disease. If the globus pallidus or the subthalamic nucleus (a relay in the indirect pathway) is partially surgically destroyed in Parkinson patients, muscular rigidity is reduced and normal movement is improved. Similarly, deep brain stimulation (DBS) of the globus pallidus inactivates it, freeing movement.
Figure 1-6 shows electrodes implanted in the brain for DBS.
Interestingly, impairments in the application of force may underlie motor disorders of skilled movements such as writer’s cramp, one of a number of impairments called selective dystonias. One such impairment, the yips, is distorted execution of skilled movements by professional athletes. For example, the yips have ended the career of many professional golfers by ruining the player’s swing (Belton et al., 2014).
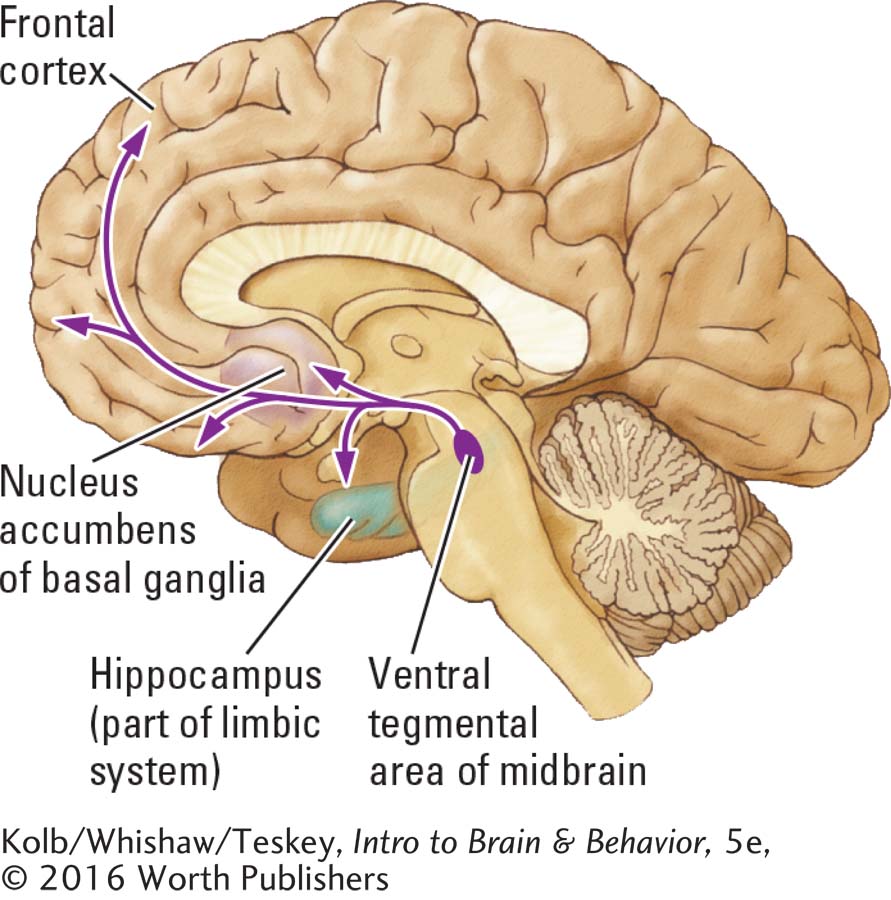
Another structure in the basal ganglia, the nucleus accumbens, is also called the ventral striatum, because it is the most ventral basal ganglia nucleus. The nucleus accumbens receives projections from dopamine cells of the ventral tegmental area, a nucleus just medial to the substantia nigra in the midbrain. Called the mesolimbic dopamine pathway, it is a part of a loop that aids our perception of cues signaling reward.
Cerebellum and Movement Skill
Musicians have a saying: “Miss a day of practice and you’re OK, miss two days and you notice, miss three days and the world notices.” Apparently, changes take place in the brain when we practice or neglect to practice a motor skill. The cerebellum may be the affected component of the motor system. Whether the skill is playing a musical instrument, pitching a baseball, or texting, the cerebellum is critical for acquiring and maintaining motor skills.
Anatomy of the Cerebellum
The elephant’s cerebellum, by contrast, contains 98.5% of its neurons. Details in Focus 1-3.
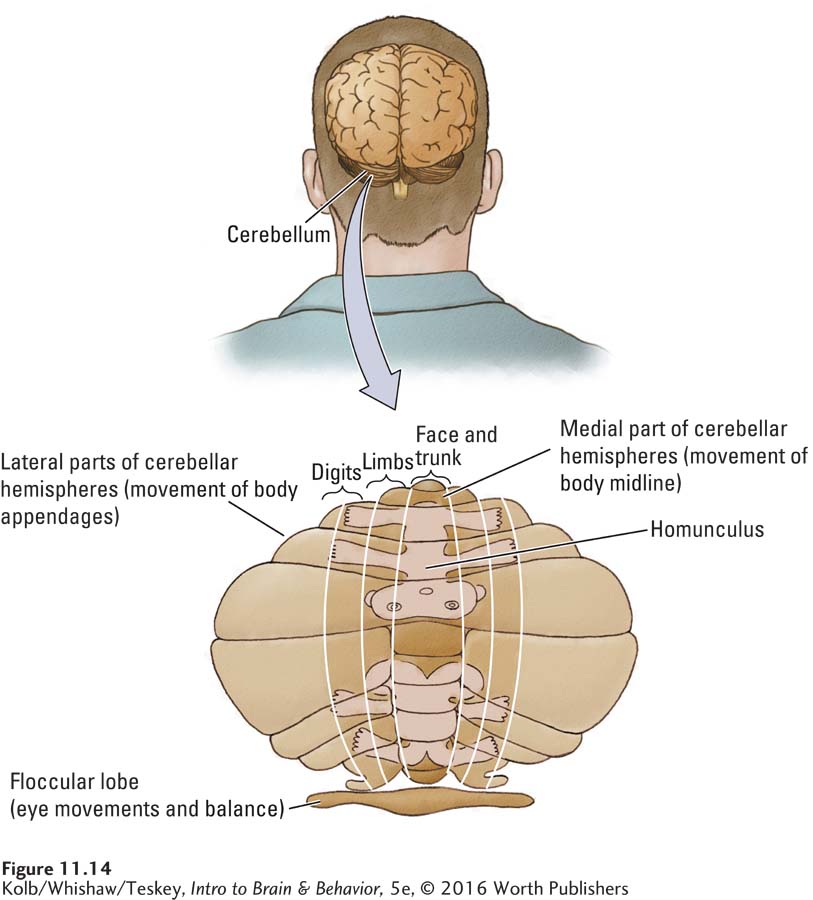
The cerebellum, a large and conspicuous part of the motor system, sits atop the brainstem, clearly visible just behind the cerebrum, and like the cerebrum, is divided into two hemispheres (Figure 11-14). A small lobe, the flocculus, projects from its ventral, or inferior, surface. Although smaller than the cerebrum, the cerebellum has many more gyri and sulci, and for all us primates, contains about four times as many neurons as does the cortex.
376
As Figure 11-14 shows, the cerebellum can be divided into several regions, each specialized for an aspect of motor control. At its base, the flocculus receives projections from the middle ear vestibular system, described in Section 11-4, and takes part in controlling balance. Many projections from the flocculus go to the spinal cord and to the motor nuclei that control eye movements.
Just as the motor cortex has a homuncular organization and multiple homunculi, the cerebellar hemispheres have at least two, as shown in Figure 11-14. The medial part of each homunculus controls the face and the body’s midline. The more lateral parts connect to motor cortex areas associated with movements of the limbs, hands, feet, and digits. Pathways from the cerebellar hemispheres project to nuclei at the interface of the cerebellum and spinal cord. These in turn project to other brain regions, including the motor cortex.
To summarize the cerebellum’s topographic organization, the midline of the homunculus is represented in its central part; the limbs and digits are represented in the lateral parts. Tumors or damage to midline areas of the cerebellum disrupt balance, eye movement, upright posture, and walking but do not substantially disrupt other movements such as reaching, grasping, and using the fingers. A person with medial damage to the cerebellum may, when lying down, show few symptoms. Damage to lateral parts of the cerebellum disrupts arm, hand, and finger movements much more than movements of the body’s trunk.
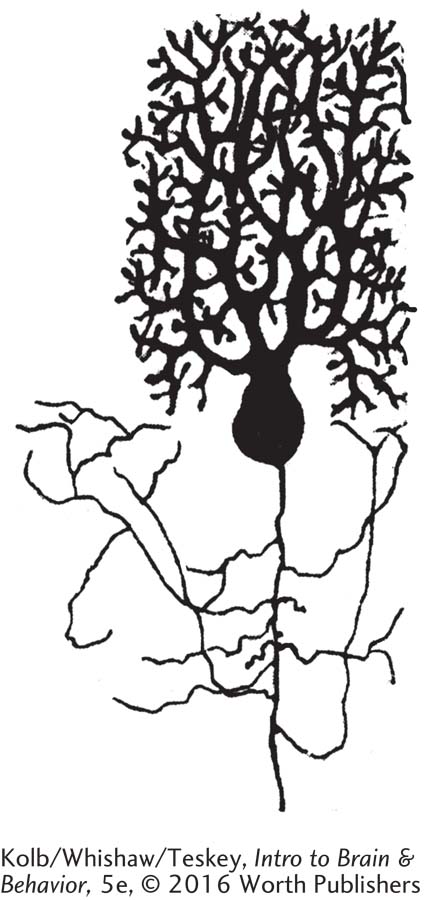
The arrangement and connections of the cerebellum are built to a common plan. The cerebellar cortex consists of three layers of cells, with the distinctive Purkinje cells forming the second layer. Purkinje cells are the output cells of the cerebellum. This plan suggests that the cerebellum has a common function with respect to its control over other motor system regions.
How the Cerebellum Improves Movement Control
Attempts to understand how the cerebellum controls movement have centered on two major ideas. Damage to the cerebellum (1) does not abolish any movement but (2) does disrupt the timing and execution of movement. Thus, the cerebellum must regulate the timing and flow of movement as required for the situation.
377
Tom Thach (2007), in an intriguing experiment, illustrates how the cerebellum helps make the adjustments needed to keep movements accurate. Control participants and subjects with cerebellar damage threw darts at a target, as shown in the Procedure section of Experiment 11-4. After a number of throws that allowed them to become reasonably accurate, both groups donned glasses containing wedge-
EXPERIMENT 11-4
Question: Does the cerebellum help to make adjustments required to keep movements accurate?
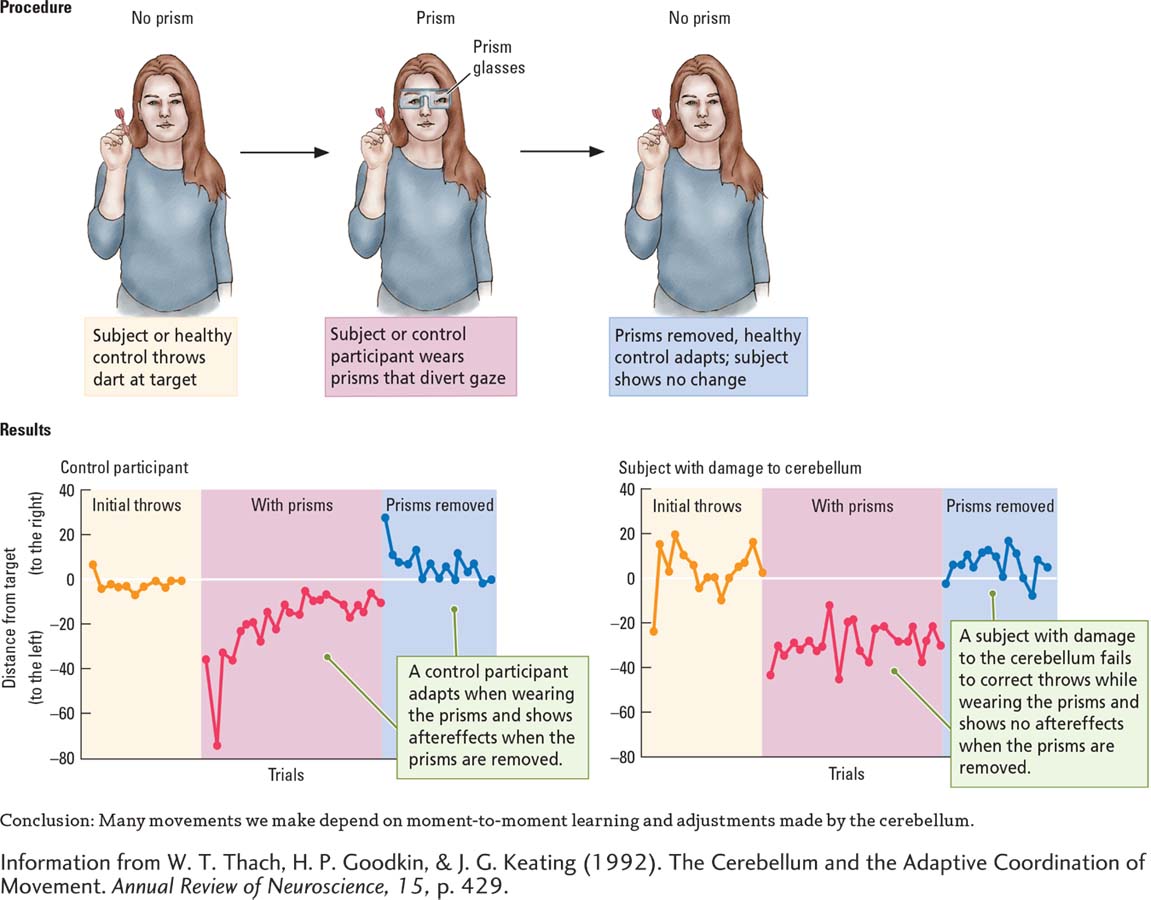
Both groups showed this initial distortion in aim. But then came an important difference, graphed in the Results section of Experiment 11-4. When controls saw the dart miss the mark, they adjusted each successive throw until reasonable accuracy was restored. In contrast, subjects with cerebellar damage could not correct for this error. Time after time, they missed the target far to the left.
Next, the controls removed the prism glasses and threw a few more darts. Again, a significant difference emerged. The first dart thrown by each participant was much too far to the right (owing to the previous adjustment they had learned to make), but soon each one adjusted once again until his or her former accuracy was regained.
378
In contrast, subjects with damage to the cerebellum showed no aftereffects of wearing the prisms; they had never compensated for the glasses to begin with. This experiment suggests that many movements we make—
To examine the role of learning in this task, monkeys were trained on a similar task to point a finger to a target on a computer screen. Once they had mastered the task, they were required to perform it with prism glasses. The monkeys displayed a displacement of pointing and an aftereffect when the prism glasses were removed.
After 30 days of training with and without prisms, displacement and aftereffect disappeared, and the monkeys were immediately accurate when wearing the prisms and after they were removed. When the arm region of the cerebellar homunculus was then anesthetized with lidocaine, a local anesthetic agent, pointing without prisms was normal, but the learned adaptation to prisms was abolished. This experiment illustrates that the cerebellum is responsible not only for online adjustments of movement but also for learning relatively permanent movement skills (Norris et al., 2011). Using a somewhat similar task, Hashimoto and associates (2015) obtained similar results using humans who have cerebellar damage.
To better understand how the cerebellum improves motor skills by adjusting movements, consider the model charted in Figure 11-15. Imagine throwing a dart. You aim at the bull’s-
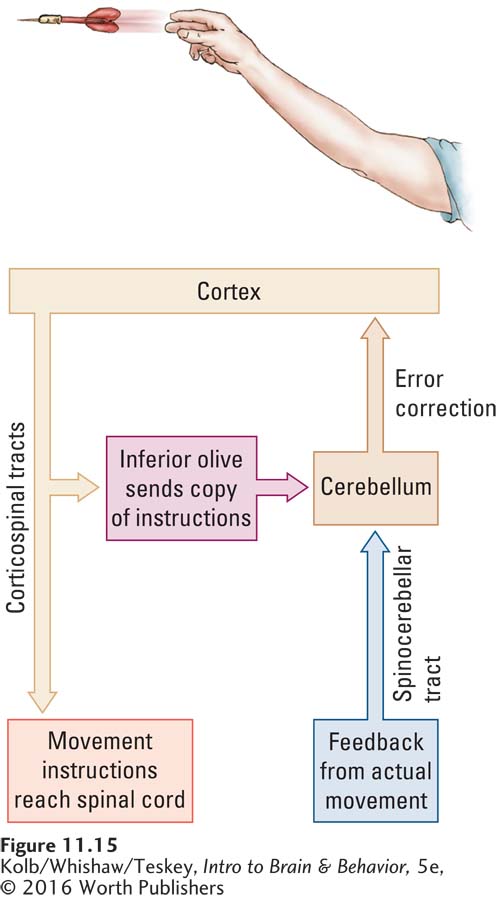
The model illustrates that the cortex sends instructions to the spinal cord to throw a dart at the target. A copy of the same instructions is sent to the cerebellum through the inferior olive, a nucleus in the brainstem that projects to the cerebellum. Then, when you throw the dart, the sensory receptors in your arm and shoulder code the actual movement you make and send a message about it back to the cerebellum through the spinocerebellar tract. The cerebellum now has information about both versions of the movement—
11-3 REVIEW
Basal Ganglia, Cerebellum, and Movement
Before you continue, check your understanding.
Question 1
The ____________ contribute to motor control by adjusting the associated with each movement.
Question 2
Damage to the basal ganglia results either in unwanted involuntary ____________ movements (too much force exerted) or in such ____________ rigidity that movements are difficult to perform (too little force exerted).
Question 3
The cerebellum contributes to motor control by improving movement ____________ and the learning of motor ____________.
Question 4
Describe how the cerebellum improves execution of motor skills.
Answers appear in the Self Test section of the book.