11-4 Somatosensory System Receptors and Pathways
379
The motor system produces movement, but without sensation, movement would lack direction and quickly become impaired. The somatosensory system is indispensable for movement. The body senses tell us about the physical contact we make with the world as well as how successful our physical interactions with the world are.
Somatic sensation is unique among sensory systems. For the most part, it is distributed throughout the body, not localized in the head, as are vision, hearing, taste, and smell. Somatosensory receptors found in the skin, muscles, and internal organs, including the circulatory system, feature specialized dendritic attachments on sensory neurons, or the dendrites themselves are the sensory receptors. Somatosensory neurons convey information to the spinal cord and brain. One part of the system, however, is confined to a single organ. The inner ear houses the vestibular system, which contributes to our sensations of balance and head movement.
In considering the motor system, we started at the cortex and followed the motor pathways out to the spinal cord (review Figures 11-9 and 11-10). This efferent route follows the outward flow of neural instructions regarding movement. As we explore the somatosensory system, we proceed in the opposite direction, because afferent sensory information flows inward, from the body’s sensory receptors through sensory pathways in the spinal cord to the cortex.
Somatosensory Receptors and Perception
Our body is covered with somatosensory receptors. Receptors include body hair, which is attached to the dendrites of sensory neurons. Many receptor types are embedded in surface skin, in deeper layers of the skin, and in muscles, tendons, and joints. Some receptors consist simply of a sensory neuron dendrite. Others are specialized capsules or connective tissue surrounding a dendrite, and still others include a dendrite attached to the base of a hair.
The density of somatosensory receptors, which varies greatly throughout the body, determines sensitivity to stimulation. Body parts that are highly sensitive to touch—
Humans have two kinds of skin, hairy skin and glabrous skin, which includes the palms of the hands and feet, lips, and tongue. Glabrous skin is hairless and exquisitely sensitive to a wide range of stimuli. It covers the body parts that we use to explore objects—
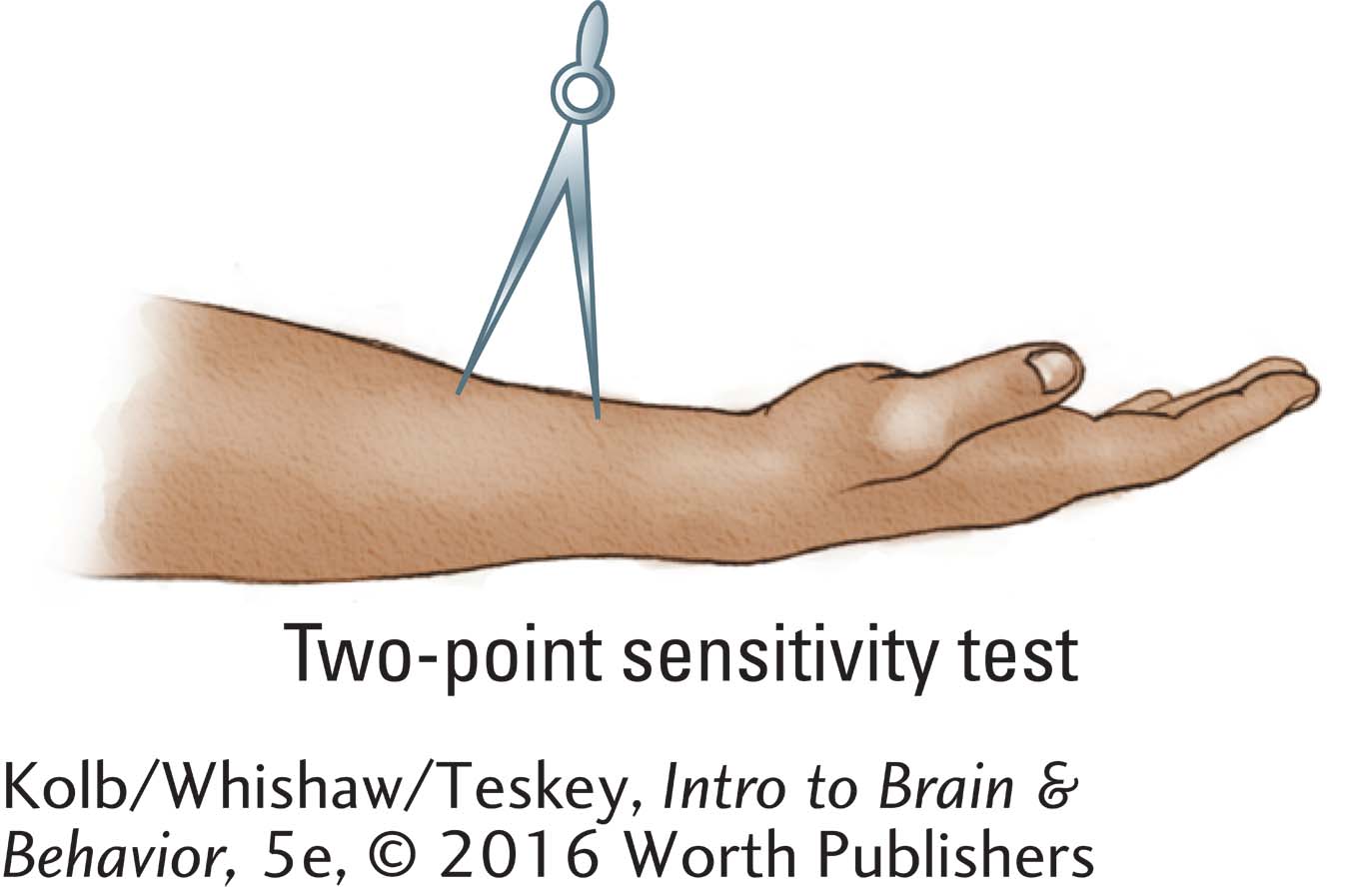
The skin’s touch sensitivity is often measured with a two-
On hairy skin, two-
Classifying Somatosensory Receptors
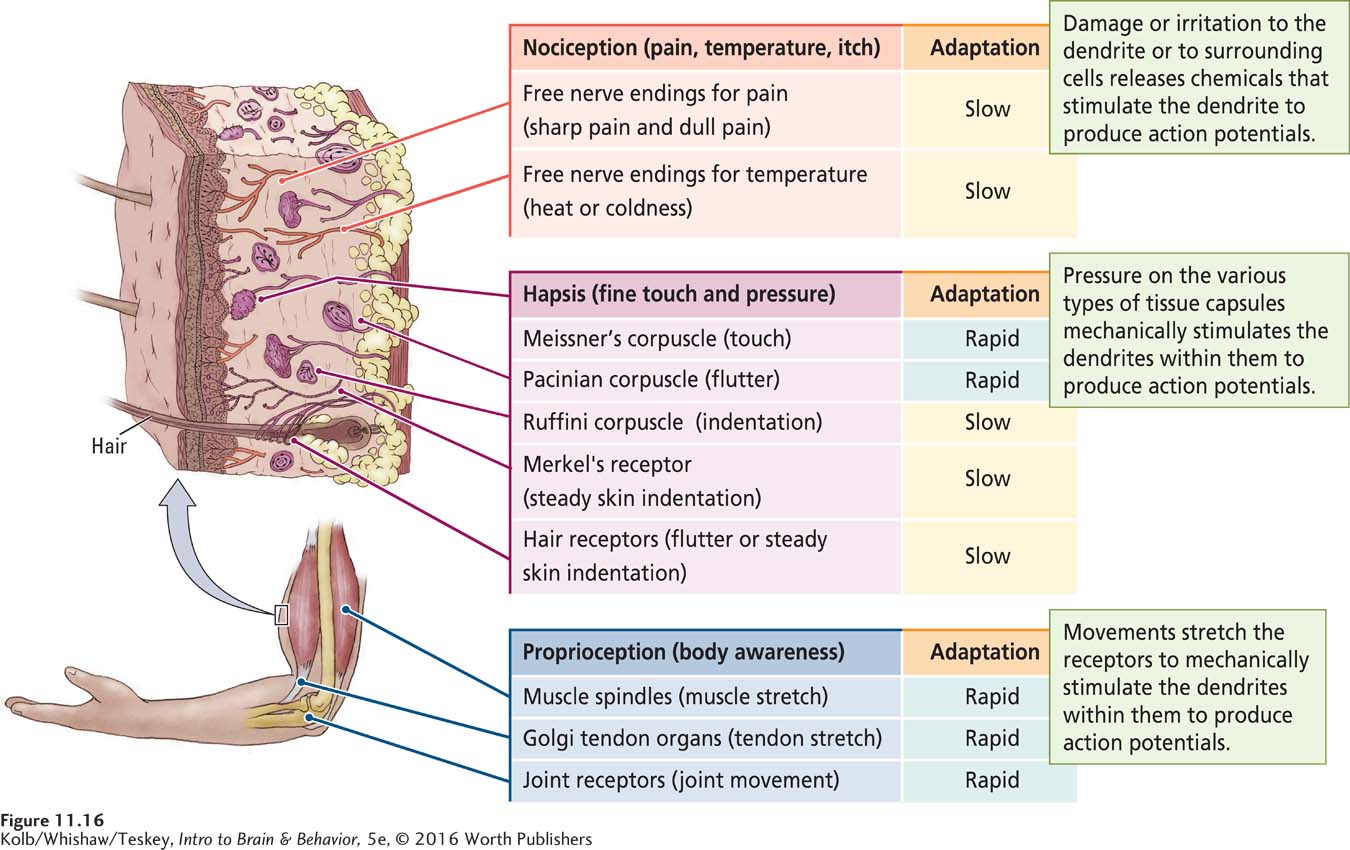
The varied types of somatosensory receptors in the human body may total as many as 20 or more, but they can all be classified into the three functional groupings illustrated in Figure 11-16 as perceptions of irritation, pressure, or movement.
380
IRRITATION Nociception is the perception of pain, temperature, or itch. Most nociceptors are free nerve endings, the tips of sensory neuron dendrites, as diagrammed at the top of Figure 11-16. When damaged or irritated, these endings secrete chemicals, usually peptides, that stimulate the nerve to produce an action potential. The action potential then conveys a message about pain, temperature, or itch to the central nervous system.
PRESSURE Hapsis (from the Greek for touch) is the ability to discriminate objects on the basis of touch. Haptic receptors enable us to perceive fine touch and pressure and to identify objects that we touch and grasp. Haptic receptors occupy both superficial and deep skin layers and are attached to body hairs as well.
Figure 4-25 illustrates the cellular processes at work in a sensory neuron dendrite when a touch receptor is activated.
As diagrammed in the center of Figure 11-16, haptic receptors consist of a dendrite attached to a hair, to connective tissue, or to a dendrite encased in a capsule of tissue. Mechanical stimulation of the hair, tissue, or capsule activates special ion channels on the dendrite, which in turn initiate an action potential. Differences in the tissue forming the capsule determine the kinds of mechanical energy transduced by the haptic receptor to the nerve. For example, pressure that squeezes the capsule of a Pacinian corpuscle is the necessary stimulus for initiating an action potential conveying pressure information. Displacement of a hair is a necessary stimulus for initiating action potentials conveying some other types of touch information.
MOVEMENT Proprioception, or body awareness, is the perception of body location and movement. Proprioceptors are encapsulated nerve endings sensitive to the stretch of muscles and tendons and the movement of joints. In the Golgi tendon organ shown at the bottom of Figure 11-16, for instance, an action potential is triggered when the tendon moves, stretching the receptor attached to it.
Duration of Receptor Response
Somatosensory receptors are specialized to tell us when a sensory event occurs and/or whether it is still occurring. Information about when a stimulus occurs is handled mainly by rapidly adapting receptors, which activate neurons when stimulation begins and when it ends. As shown in Figure 11-16, haptic receptors that respond to touch (Meissner corpuscles), to fluttering sensations (Pacinian corpuscles), and to vibration (Ruffini corpuscles) all are rapidly adapting receptors.
381
In contrast, slowly adapting receptors activate neurons as long as a sensory event is present: they detect whether a stimulus is still occurring. For instance, after you have put on an article of clothing and become accustomed to how it feels, only slowly adapting haptic receptors (such as Merkel receptors and hair receptors) remain active.
The difference between a rapidly adapting and a slowly adapting receptor rests on two factors: how the receptor is stimulated and how the ion channels in the membrane of its dendrite respond to mechanical stimulation. The stimulation may be sharp or cold, fluttery or deep, a stretch or a swerve.
Posterior Root Ganglion Neurons
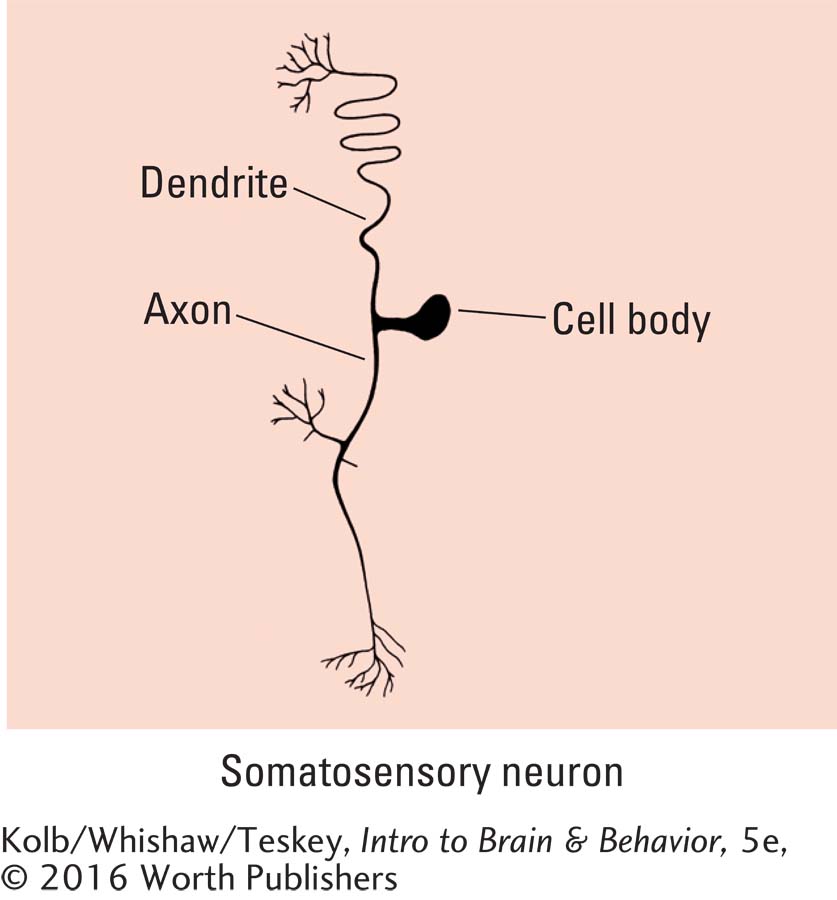
The dendrites that carry somatosensory information into the CNS belong to neurons whose cell body is just outside the spinal cord in posterior root ganglia. Their axons enter the spinal cord. As illustrated in Figure 11-17, such a posterior root ganglion neuron contains a single long dendrite. Only the tip is responsive to sensory stimulation. This dendrite is continuous with the somatosensory neuron’s axon, which enters the spinal cord. The somatosensory cell body sits to one side of this long pathway.
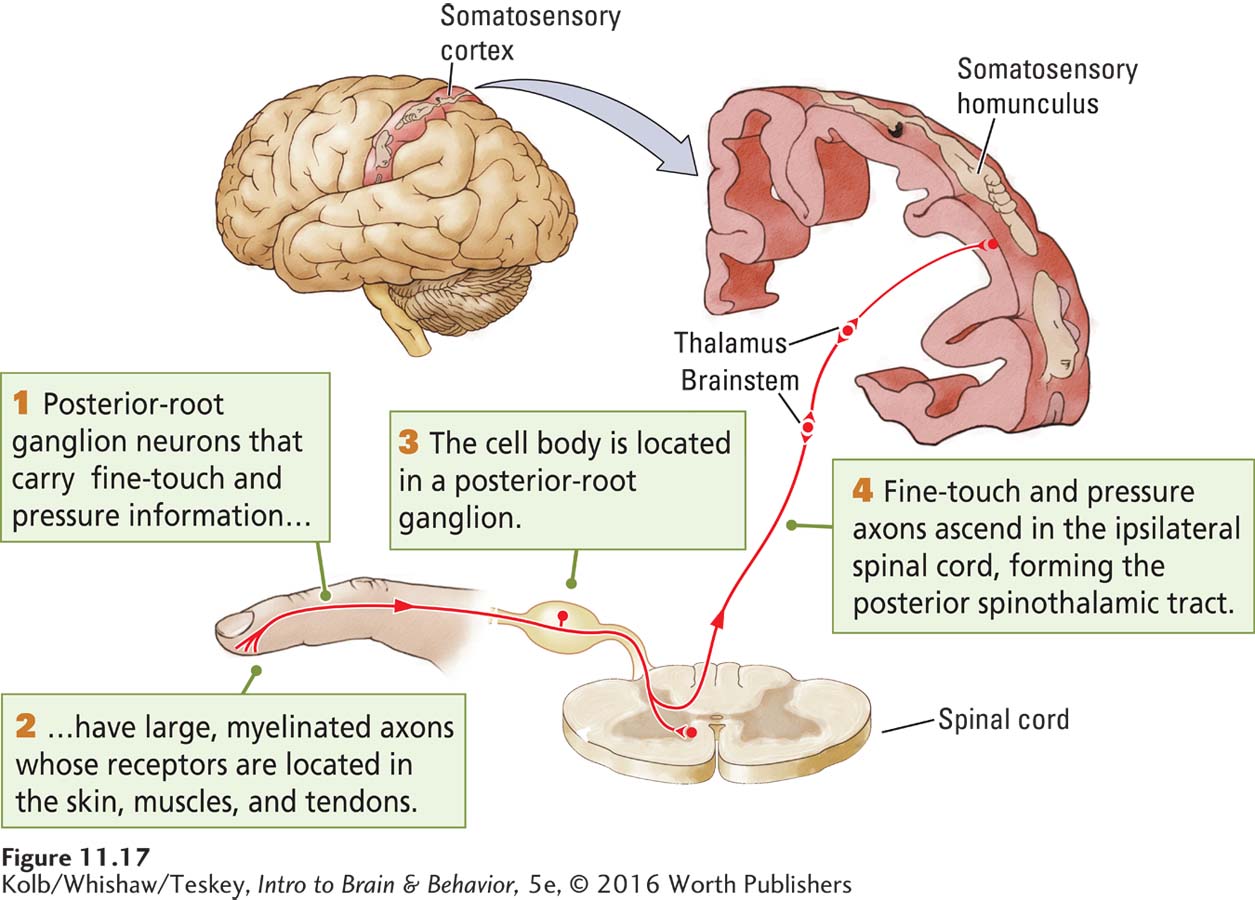
Every spinal cord segment is flanked by a posterior root ganglion that contains many types of neurons. Each type responds to a particular kind of somatosensory information. Within the spinal cord, the axons of posterior root ganglion neurons may synapse with other neurons or continue to the brain or do both.
The axons of posterior root ganglion neurons vary in diameter and myelination. These structural features are related to the kind of information the neurons carry. Proprioceptive information (location and movement) and haptic information (touch and pressure) are carried by posterior root ganglion neurons that have large, well-
Because of their size and myelination, the larger neurons carry information faster than the smaller neurons do. One possible reason proprioceptive and haptic neurons are designed to carry messages quickly is that their information requires rapid response. Imagine that you’ve touched a hot stove. A myelinated pain fiber activates and instructs the hand to withdraw quickly. A nonmyelinated pain fiber, less hurried, will let you know for some time afterward that your finger has been burned.
Myelin is the fatty coating around axons, formed by glial cells, that speeds neurotransmission. See Section 3-1.
Disruption of Posterior Root Ganglion Function
382
We can support the claim that sensory information is essential for movement by describing what happens when posterior root ganglion cells do not function. A clue comes from a visit to the dentist. If you have ever had a tooth frozen for dental work, you have felt the strange effect of losing sensation on one side of your face. Not only do you lose pain perception but you also seem to lose the ability to move your facial muscles properly, making it awkward to talk and smile and downright dangerous to chew. Similarly, if your hands or mouth become terribly cold, disrupting receptor function, talking or making hand movements becomes difficult. So even though only the sensory nerves are blocked, movement is affected as well.
In much the same way, sensory nerve damage affects both sensory perceptions and motor abilities. John Rothwell and his coworkers (1982) described a patient, G. O., who was deafferentated (had lost afferent sensory fibers) by a disease that destroyed somatosensory posterior root ganglion neurons. G. O. received no sensory input from his hands. He could not, for example, feel when his hand was holding something.
However, G. O. could still accurately produce a range of finger movements, and he could outline figures in the air even with his eyes closed. He could also move his thumb accurately through different distances and at different speeds, judge weights, and match movement force. Nevertheless, his hands were relatively useless to him in daily life. Although G. O. could drive his old car, he was unable to learn to drive a new one. He was also unable to write, to fasten shirt buttons, and to hold a cup.
G. O. began movements quite normally, but as he proceeded, the movement patterns gradually fell apart, ending in failure. Part of G. O.’s difficulties lay in maintaining muscle force for any time. When he tried to carry a suitcase, he would quickly drop it unless he continually looked down to confirm that he was carrying it. Clearly, although only G. O.’s sensory neurons were damaged, he also had severe motor disability, including inability to learn new motor skills.
Disruption of Body Awareness
Oliver Sacks’s research informed scientific understanding of conditions as diverse as Parkinson disease (Focus 5-3 and Section 16-3), ventral visual stream injury (Section 9-4), and thinking (Section 15-1).
Movement abnormalities also result from more selective damage to neurons that carry proprioceptive information about body location and movement. Neurologist Oliver Sacks (1998) gives a dramatic example in his description of a patient, Christina, whose proprioceptive sensory fibers throughout her body were damaged after she took megadoses of vitamin B6. Christina was left with little ability to control her movements and spent most of each day lying prone. Here is how she describes what a loss of proprioception means:
“What I must do then,” she said slowly, “is use vision, use my eyes, in every situation where I used—
Clearly, Christina’s motor system is intact, but with no sense of where in space her body is and what it is doing, she is almost completely immobilized. Jonathan Cole (1995) described the case of Ian Waterman, who lost proprioception after a presumed viral infection at age 19. He is the only person reported to have learned how to move again, and this relearning took years. He was even able to drive. All his regained movement was mediated by vision, however, without which he was as helpless as Christina.
Somatosensory Pathways to the Brain
Ipsilateral connections lie on the side of the body on which they enter; contralateral connections lie on the side opposite.
As the axons of somatosensory neurons enter the CNS in the spinal cord, they divide, forming two pathways to the brain. The haptic-
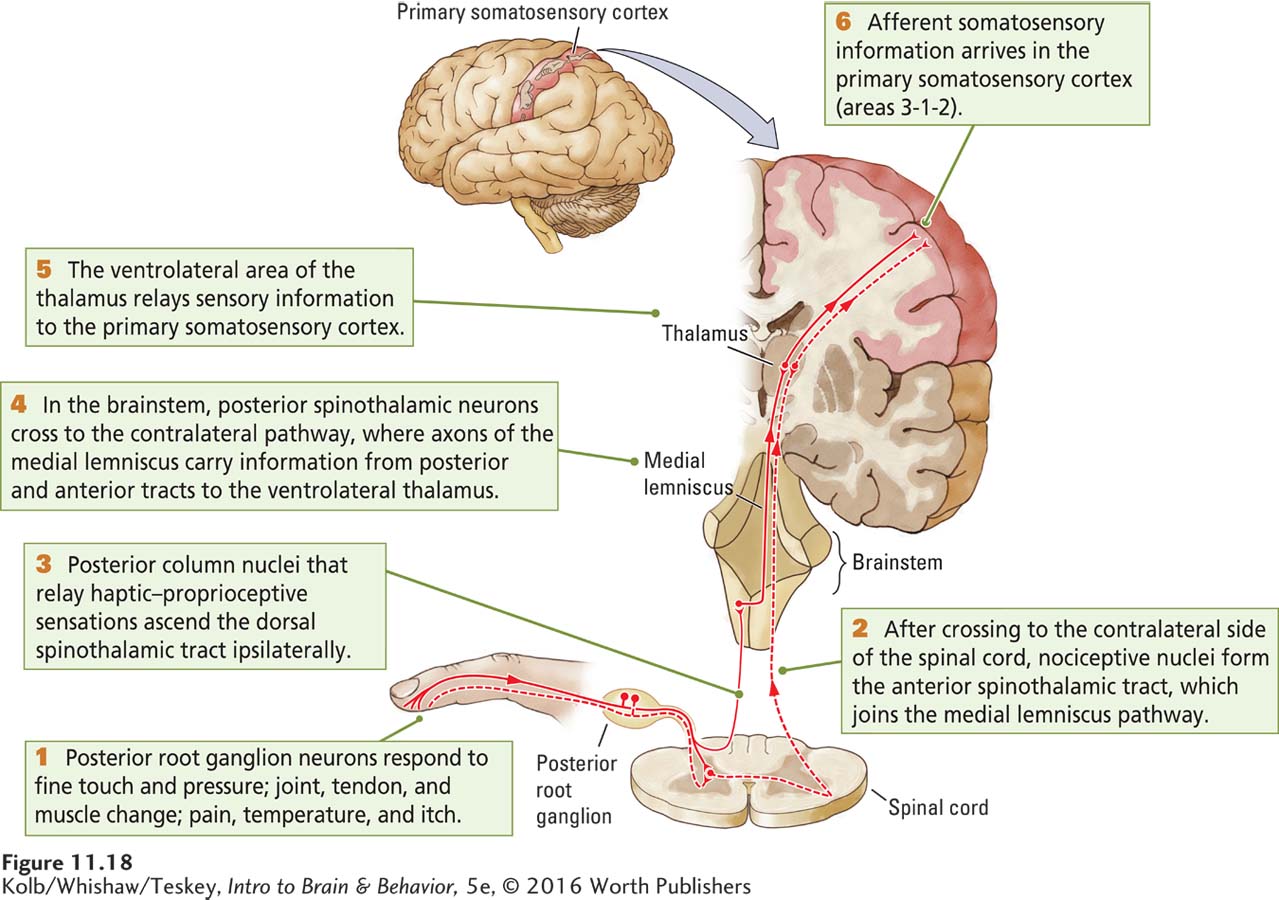
The Posterior Spinothalamic Tract
383
Haptic-
These posterior column axons synapse in the ventrolateral thalamus, the part of the thalamus that sends afferent information about body senses on to the somatosensory cortex. Although ventrolateral thalamic neurons send most of their axons to the somatosensory cortex, some do go to the motor cortex. Thus, three relay neurons are required to carry haptic-
The Anterior Spinothalamic Tract
Most nociceptive axons take a different route to the brain. As shown in Figure 11-18, they first synapse with neurons in the anterior part of the spinal cord’s gray matter, and in turn, these neurons send their axons across to the other side of the spinal cord. There they form the anterior spinothalamic tract, which carries afferent information about pain, temperature, and itch to the thalamus. This tract joins the medial lemniscus in the brainstem to continue on to the ventrolateral thalamus. The thalamic neurons project to the somatosensory cortex. So again, conveying information to the cortex requires three groups of relay neurons: posterior root neurons, spinal cord gray matter neurons, and ventrolateral thalamic neurons.
Effects of Unilateral Somatosensory System Damage
384
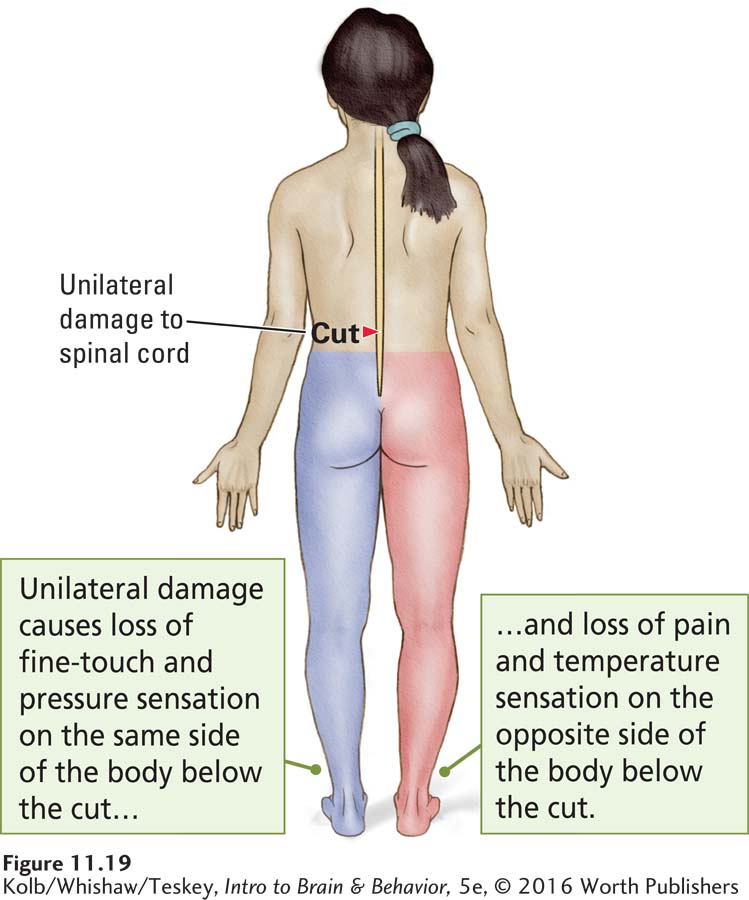
Unilateral damage dissociates the functions of the two somatosensory pathways—
Damage to the posterior roots produces global somatosensory deficits in a particular part of the body. In contrast, as illustrated in Figure 11-19, after unilateral spinal cord injury, loss of hapsis and proprioception occurs unilaterally, to the side of the body below where the damage occurred. Loss of nociception occurs contralaterally. This occurs because hapsis and proprioception take different routes through the spinal cord. Unilateral damage at the points where the pathways merge in the brainstem, thalamus, and cortex again affects hapsis, proprioception, and nociception together, because these parts of the pathways again lie in close proximity.
Spinal Reflexes
Not only do somatosensory nerve fibers convey information to the cortex, they also participate in behaviors mediated by the spinal cord and brainstem. Spinal cord somatosensory axons, even those ascending the posterior columns, give off axon collaterals that synapse with interneurons and motor neurons on both sides of the spinal cord. The circuits made between sensory neurons and muscles through these connections mediate spinal reflexes.
The simplest spinal reflex is formed by a single synapse between a sensory neuron and a motor neuron. Figure 11-20 illustrates such a monosynaptic reflex, the knee jerk. It affects the quadriceps muscle of the thigh, which is anchored to the leg bone by the patellar tendon. When the lower leg hangs free and this tendon is tapped with a small hammer, the quadriceps muscle is stretched, activating the stretch-
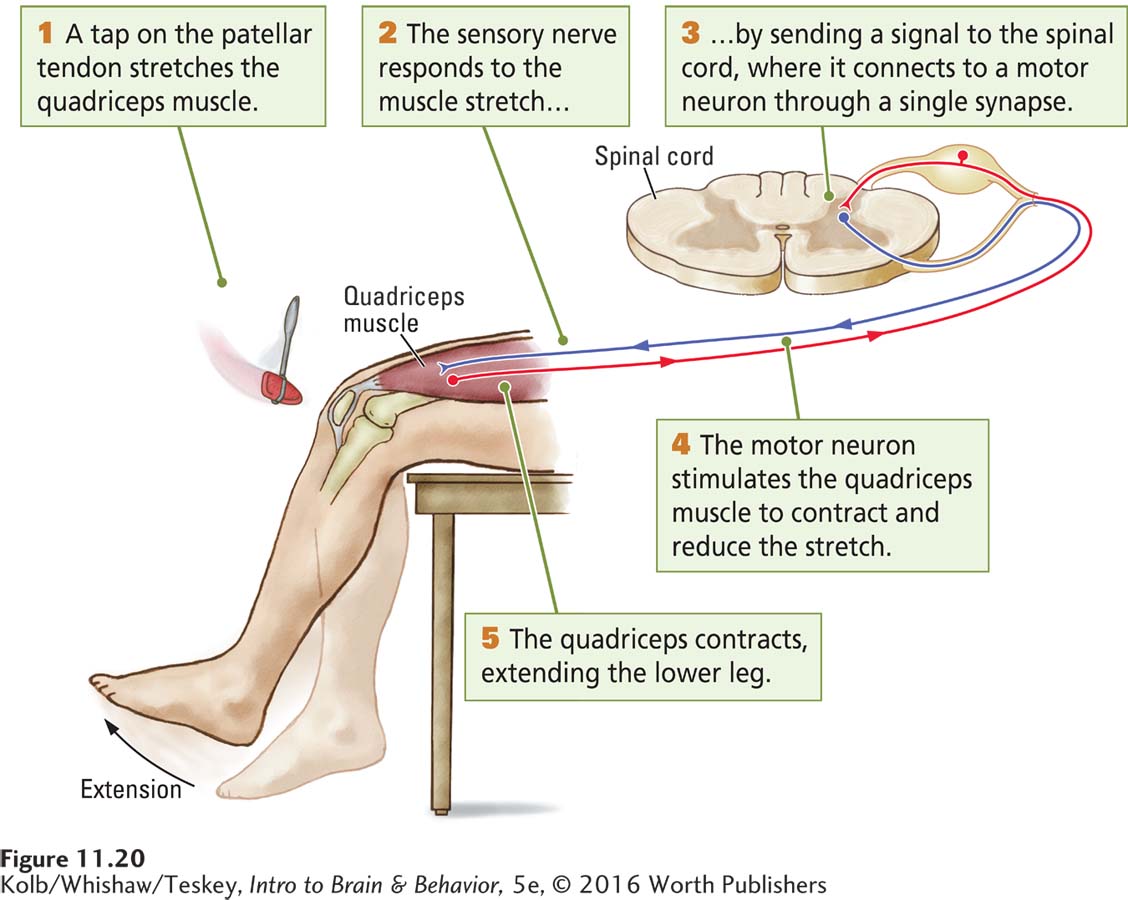
The sensory receptors then send a signal to the spinal cord through sensory neurons that synapse with motor neurons projecting to the same thigh muscle. The discharge from the motor neurons stimulates the muscle, causing it to contract to resist the stretch. Because the tap is brief, the stimulation is over before the motor message arrives, and the muscle contracts even though it is no longer stretched. This contraction pulls the leg up, producing the knee jerk reflex.
385
This simplest of reflexes entails monosynaptic connections between single sensory neurons and single motor neurons. Somatosensory neurons receiving information from the skin make much more complex connections with both interneurons and motor neurons. These multisynaptic connections are responsible for more complex spinal movements, such as those involved in standing and walking, actions that include many muscles on both sides of the body.
Feeling and Treating Pain
As many as 30 percent of physician visits involve pain symptoms, as do 50 percent of emergency room visits. People have pain as a result of acute injuries such as broken bones, chronic conditions such as cancer and arthritis, and everyday conditions such as stiff muscles from exercising. Women have pain during menstruation, pregnancy, and childbirth. The incidence of living with pain increases as people age, and for many people, pain is a constant companion.
Perceiving Pain
People can experience central pain in a part of the body that is not obviously injured. One type of central pain is phantom limb pain. As described in Research Focus 11-5, Phantom Limb Pain on page 386, phantom sensations are felt in a limb that has been lost, hence the term.
People in pain would happily dispense with it. But pain is necessary: the rare person born without pain receptors has body deformities because of failure to adjust posture and acute injuries because of failure to avoid harm.
Pain perception results from synthesizing a plethora of sensory information. There may be as many as eight kinds of pain fibers, judging from the peptides and other chemicals released by these nerves when irritated or damaged. Some of these chemicals irritate surrounding tissue, stimulating it to release other chemicals to stimulate blood flow and to stimulate the pain fibers themselves. These reactions contribute to pain, redness, and swelling at the site of an injury.
Consider itch. We may feel itchy and consequently scratch a foreign object on our body. We also frequently feel itch in the absence of an obvious stimulus. Some drugs, including opioids, enhance itch sensations in the absence of a physical stimulus at the itchy body part.
Haptic information also contributes to pain perception. For example, people can accurately report the location and characteristics of various kinds of pain, but in the absence of fine-
The anterior spinothalamic tract, illustrated in Figure 11-18, is the main pain pathway to the brain, but as many as four other pathways may carry pain information from the spinal cord to the brain. These pathways are both crossed and uncrossed and project to the reticular formation of the midbrain, where they produce arousal; to the amygdala, where they produce emotional responses typically associated with pain; and to the hypothalamus, where they activate hormonal and cardiovascular responses.
The existence of multiple pain pathways in the spinal cord makes it difficult to treat chronic pain, even by selectively cutting the anterior spinothalamic tract—
Responding to Pain
Neuronal circuits in the spinal cord allow haptic-
386
RESEARCH FOCUS 11-5
Phantom Limb Pain
Up to 80 percent of people who have had a limb amputated also endure phantom limb phenomena, including pain and other sensations and motor phantoms, such as phantom movement and cramps (Kern et al., 2009). Phantom sensations and movements are illusions that originate in the brain.
Various techniques used to minimize phantom limb pain include drug-
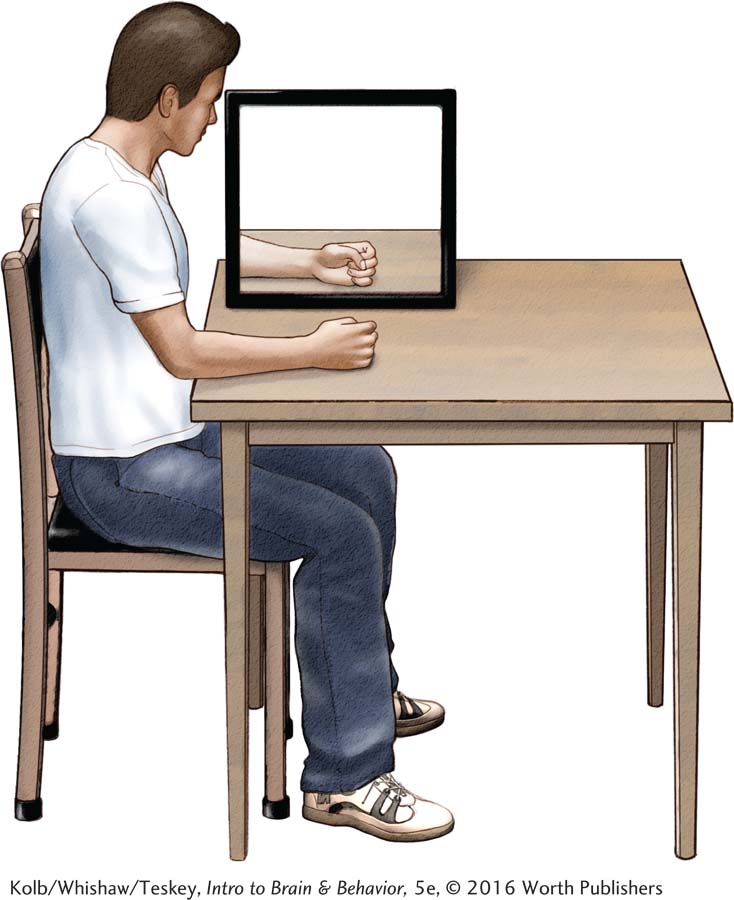
Ramachandran devised a mirror box into which a person who has lost an arm inserts the intact arm and then observes its reflection in the mirror. The reflection suggests that the missing arm is present and can be controlled, as shown in the illustration. The perception of the limb as intact counteracts phantom pain and cramps.
Inspired by Ramachandran’s mirror box, researchers have developed other illusions to suggest that a missing limb is present and can be controlled. One method uses virtual reality goggles. Another uses the so-
All of the illusions lessen phantom limb pain and cramps by suggesting that a normal limb is present. Using similar logic, Hellman and associates (2015) suggest the possibility of developing neuroprosthetic limbs that provide their users sensory information to alleviate phantoms.
Alessandria and colleagues (2011) asked whether phantom limbs and their associated sensations also occur during dreaming. They awoke sleeping participants with amputations during rapid eye movement, or REM, sleep and asked them to recount their dreams. In none of the dreams did the participants remember having an amputated limb or phantom limb sensations.
This finding suggests that the integrity of brain regions associated with a limb is preserved even though the limb is absent. When awake, the loss of moment-
A friend of ours was attacked by a grizzly bear while hiking and received 200 stitches to bind his wounds. When friends asked if it hurt to be bitten by a grizzly bear, he surprisingly answered no, explaining, “I had read the week before about someone who was killed and eaten by a grizzly bear. So I was thinking that this bear was going to eat me unless I got away. I did not have time for pain. I was fighting for my life. It was not until the next day that I started feeling pain.”
Section 6-2 notes similarities between the brain’s endogenous opioids and opioid analgesic drugs.
The primacy of our friend’s fear over his pain is related to the stress he was under. Not feeling pain in a fight-
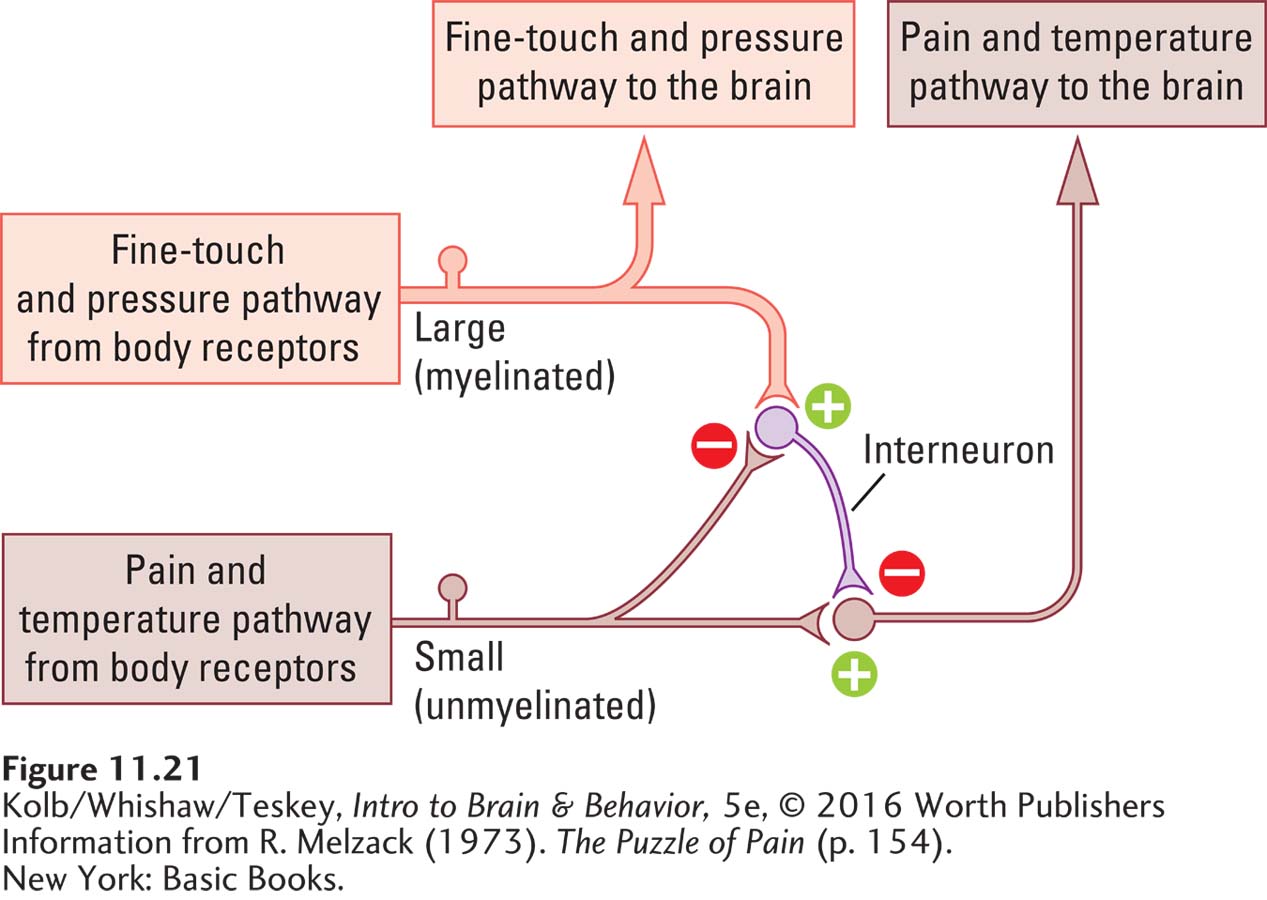
To explain both the perception of pain and how it can be suppressed in so many ways, Ronald Melzack and Patrick Wall (1965) proposed a gate theory of pain. Its essence as applied to pain perception is that activities in different sensory pathways play off against each other and so determine whether and how much pain is perceived as a result of an injury. Melzack and Wall propose that haptic-
387
A model of the pain gate, illustrated in Figure 11-21, consists of a haptic-
Treating Pain
Gate theory helps to explain how so many pain treatments work (Foster et al., 2015). When you stub your toe, for instance, you feel pain because the pain pathway to the brain is open. Rubbing the toe activates the haptic-
Similarly, a variety of pain treatments, including massage, warm water immersion, and acupuncture, may produce pain-
Figure 2-4 diagrams the triple-
One of the most successful pain treatments is injecting small amounts of morphine under the dura mater, the outer layer of the meninges. This epidural anesthesia is mediated by the action of morphine on interneurons in the spinal cord. Although morphine is a highly useful pain treatment, its effects lessen with continued use. This form of habituation may be related to changes that take place on the postsynaptic receptors of pain neurons in the spinal cord and brain.
Gate theory also suggests an explanation for the pins and needles we feel after sitting too long in one position. Loss of oxygen from reduced blood flow first deactivates the large myelinated axons that carry touch and pressure information, leaving the small unmyelinated fibers that carry pain and temperature messages unaffected. As a result, ungated sensory information flows in the pain and temperature pathway, leading to the pins and needles.
Neural circuits resembling pain gates may be located in the brainstem and cortex as well as the spinal cord. These gates help to explain how cognition and emotion influence pain and how other approaches to pain relief work. For example, researchers have found that feelings of severe pain can be lessened when people can shift their attention from the pain to other stimuli. Dentists have long used this technique by giving their patients something soothing to watch or listen to during procedures.
388
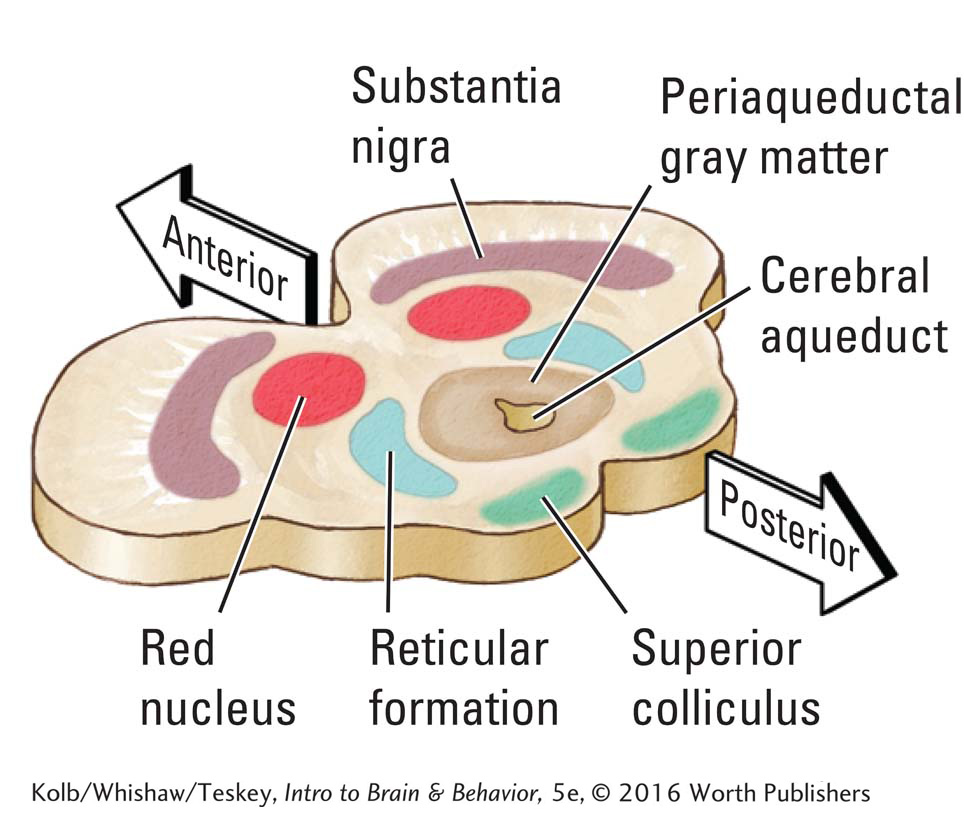
The brain can also influence the pain signal it receives from the spinal cord. The cell bodies of periaqueductal gray matter (PAG) neurons surround the cerebral aqueduct connecting the third and fourth ventricles. Electrical stimulation of the PAG is effective in suppressing pain. Neurons in the PAG produce their pain-
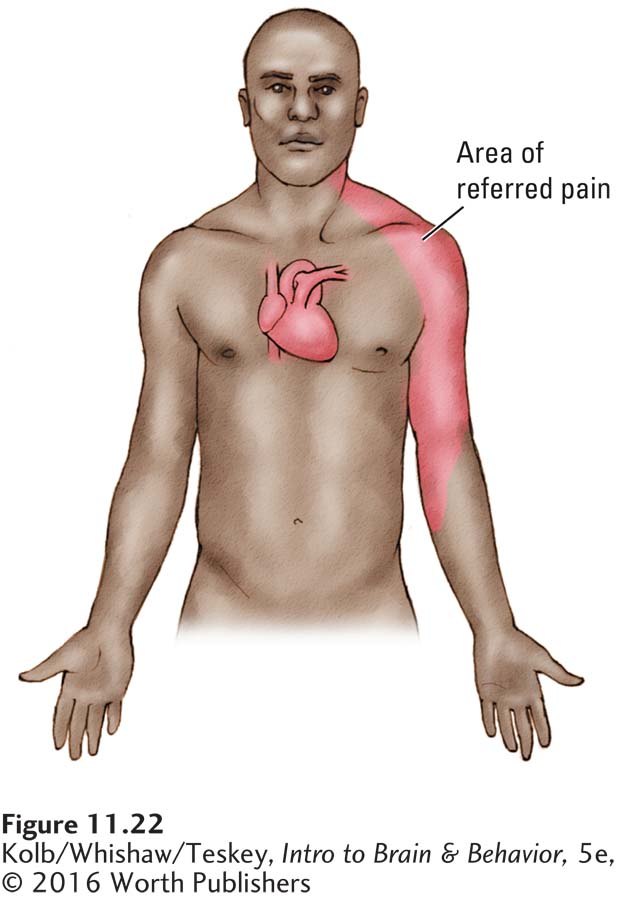
Many internal organs, including the heart, kidneys, and blood vessels, have pain receptors, but the ganglion neurons carrying information from these receptors do not have their own pathway to the brain. Instead, they synapse with spinal cord neurons that receive nociceptive information from the body’s surface. Consequently, the spinal cord neurons that relay pain and temperature messages to the brain receive two sets of signals: one from the body’s surface and the other from the internal organs.
These spinal cord neurons cannot distinguish the two sets of signals—
Vestibular System and Balance
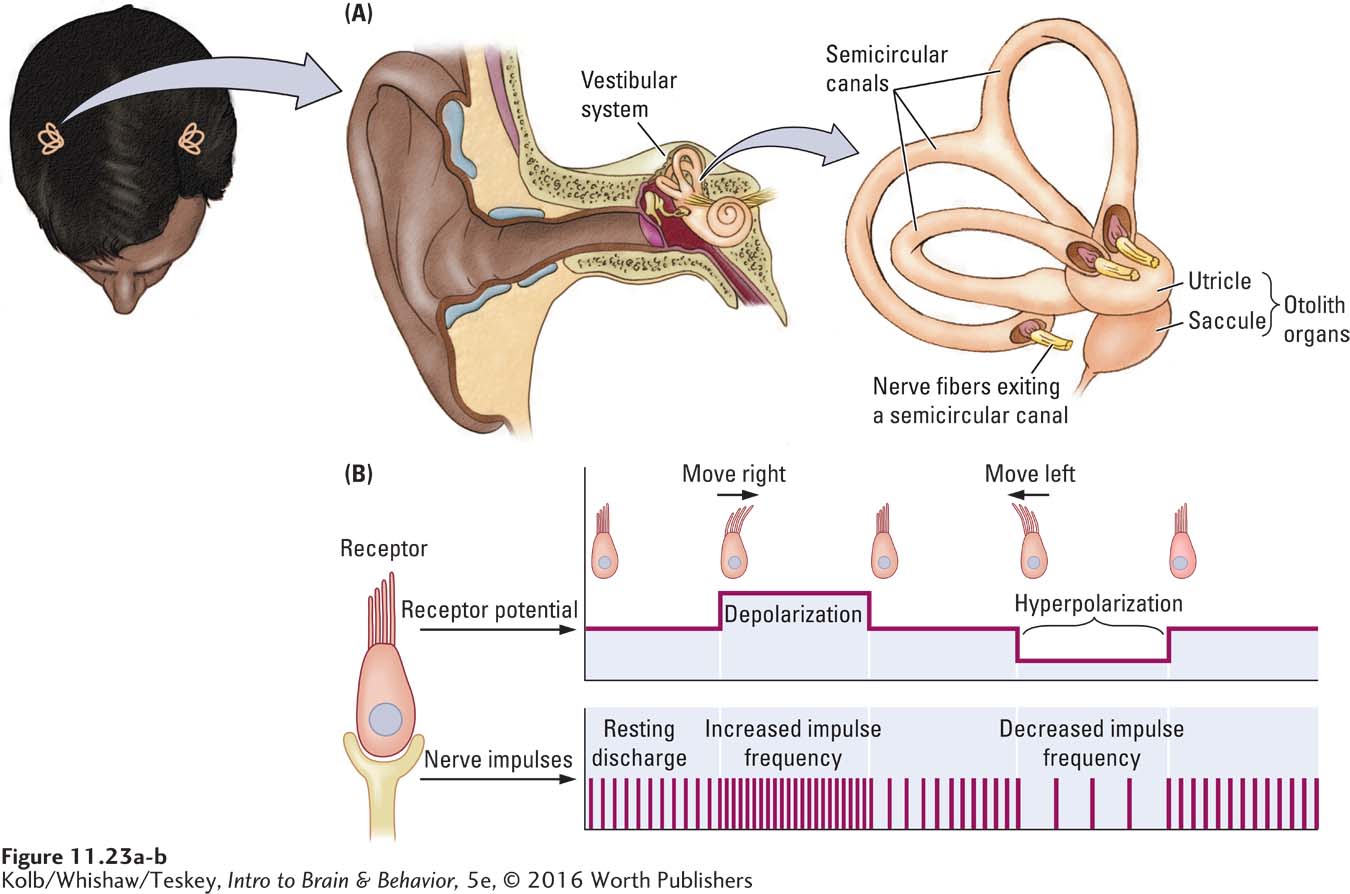
The only localized part of the somatosensory system, the vestibular system, consists of two organs, one in each inner ear. As Figure 11-23A shows, each vestibular organ consists of two groups of receptors: the three semicircular canals and the otolith organs, the utricle and the saccule. These vestibular receptors do two jobs: (1) they tell us the position of the body in relation to gravity and (2) they signal changes in direction and speed of head movement.
Vestibular hair cells work on the same principles as cochlear hair cells, which mediate hearing. See Section 10-2.
In Figure 11-23A the semicircular canals are oriented in three planes that correspond to the three dimensions in which we move through space. Each canal furnishes information about movement in its particular plane. The semicircular canals are filled with a fluid called endolymph. Immersed in the endolymph is a set of hair cells.
Cranial nerve 8 conveys both hearing and balance information to the brain. See Figure 2-27.
When the head moves, the endolymph also moves, pushing against the hair cells and bending the cilia at their tips. The force of the bending is converted into receptor potentials in the hair cells that send action potentials over auditory vestibular nerve axons to the brain. These axons are normally quite active: bending the cilia in one direction increases receptor potentials, consequently increasing vestibular nerve axon activity; bending them in the other direction decreases vestibular afferent axon activity. These responses are diagrammed in Figure 11-23B. Typically, when the head turns in one direction, the receptor message on that side of the body increases neuronal firing. The message on the body’s opposite side leads to decreased firing.
The utricle and saccule lie stacked just beneath the semicircular canals, as shown at the right in Figure 11-23A. These organs also contain hair cells, but the receptors are embedded in a gelatinous substance that contains otoconia (sing. otoconium), small crystals of the salt calcium carbonate. When you tilt your head, the gelatin and otoconia press against the hair cells, bending them. The mechanical action of the hair bending modulates the rate of action potentials in vestibular afferent axons that convey messages about the head’s position in three-
The receptors in the vestibular system tell us about our location relative to gravity, about acceleration and deceleration of our movements, and about changes in movement direction. They also allow us to ignore the otherwise destabilizing influence that our movements might have on us. When you are standing on a moving bus, for example, even slight movements of the vehicle could potentially throw you off balance, but they do not. Similarly, when you move, you easily avoid tipping over, despite the constant shifting of your body weight. Your vestibular system enables your stability.
389
To demonstrate vestibular receptors’ role in helping you to compensate for your own movements, try this experiment. Hold your hand in front of you and shake it. Your hand appears blurry. Now shake your head instead of your hand, and the hand remains in focus. Compensatory signals from your vestibular system allow you to see the hand as stable even though you are moving around.
Vertigo (from the Latin for spinning), a sensation of spinning when one is not moving, a dysfunction of the inner ear, can be accompanied by nausea as well as difficulty maintaining balance while walking. A common way to induce vertigo is to spin, as children do when playing. Vertigo can also occur from looking down from a height or looking up at a tall object and as one is simply standing up or sitting down. One intoxicating effect of alcohol is vertigo. Ménière disease, named after a French physician, is a disorder of the inner ear resulting in vertigo and loss of balance (Lopez-
11-4 REVIEW
Somatosensory System Receptors and Pathways
Before you continue, check your understanding.
Question 1
Body senses contribute to the perception of ____________ (touch and pressure), ____________ (location and movement), and ____________ (temperature, pain, itch).
Question 2
Haptic-
Question 3
The two tracts interact in the spinal cord to regulate pain perception via a ____________.
Question 4
In the midbrain, the ____________ suppresses pain by activating neuromodulatory circuits that inhibit pain pathways.
Question 5
The only localized somatosensory system is the ____________ system, which helps us to maintain ____________ by signaling information about the head’s position and our movement through space.
Question 6
Explain how proprioception acts as the “eyes of the body.”
Answers appear in the Self Test section of the book.