11-5 Exploring the Somatosensory Cortex
390
Somatosensory neurons do more than convey sensation to the brain: they enable us to perceive things that we describe as pleasant or unpleasant, the shape and texture of objects, the effort required to complete tasks, and even our spatial environment. They also play a central role in movement. Figure 11-24 illustrates the two main somatosensory areas in the cortex.
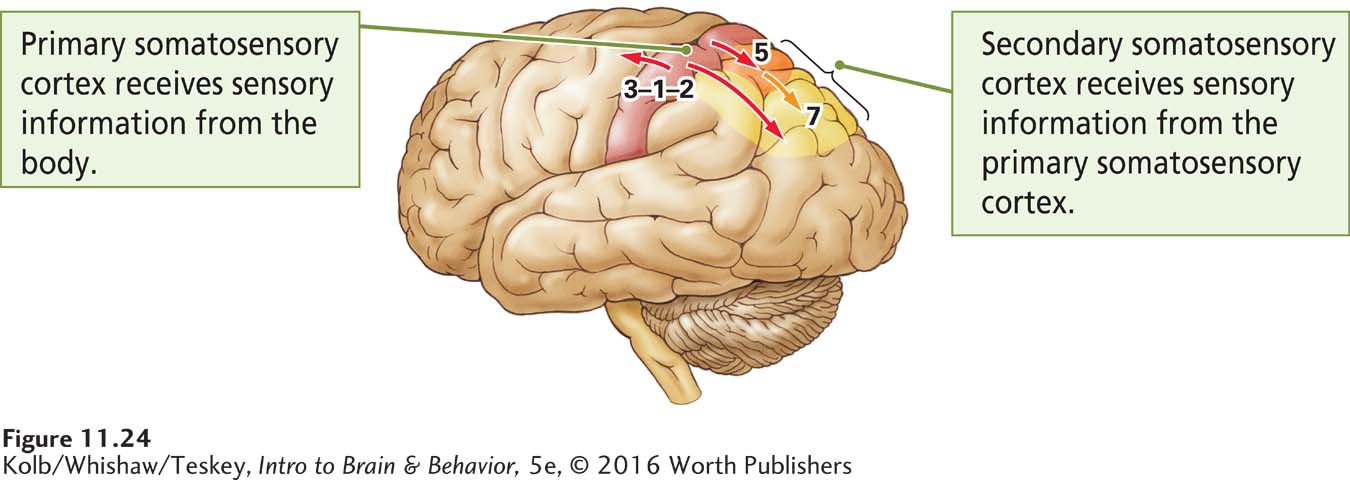
Korbinian Brodmann numbered these areas more than a century ago on his map of the cortex (see Figure 2-23).
The primary somatosensory cortex (S1), which receives projections from the thalamus, consists of Brodmann areas 3-
The secondary somatosensory cortex (Brodmann areas 5 and 7, shaded orange and yellow in Figure 11-24) is located in the parietal lobe just behind S1. The secondary somatosensory cortex (S2) refines perceptual constructions in relation to potential movement and sends information to the frontal cortex.
Somatosensory Homunculus
In his studies of human patients undergoing brain surgery, Wilder Penfield electrically stimulated the somatosensory cortex and recorded patients’ responses. Stimulation at some sites elicited sensations in the foot; stimulation of other sites produced sensations in a hand, the trunk, or the face. By mapping these responses, Penfield was able to construct a somatosensory homunculus in the cortex, shown in Figure 11-25A. The sensory homunculus looks nearly identical to the motor homunculus shown in Figure 11-6 in that the most sensitive areas of the body are accorded relatively large cortical areas.
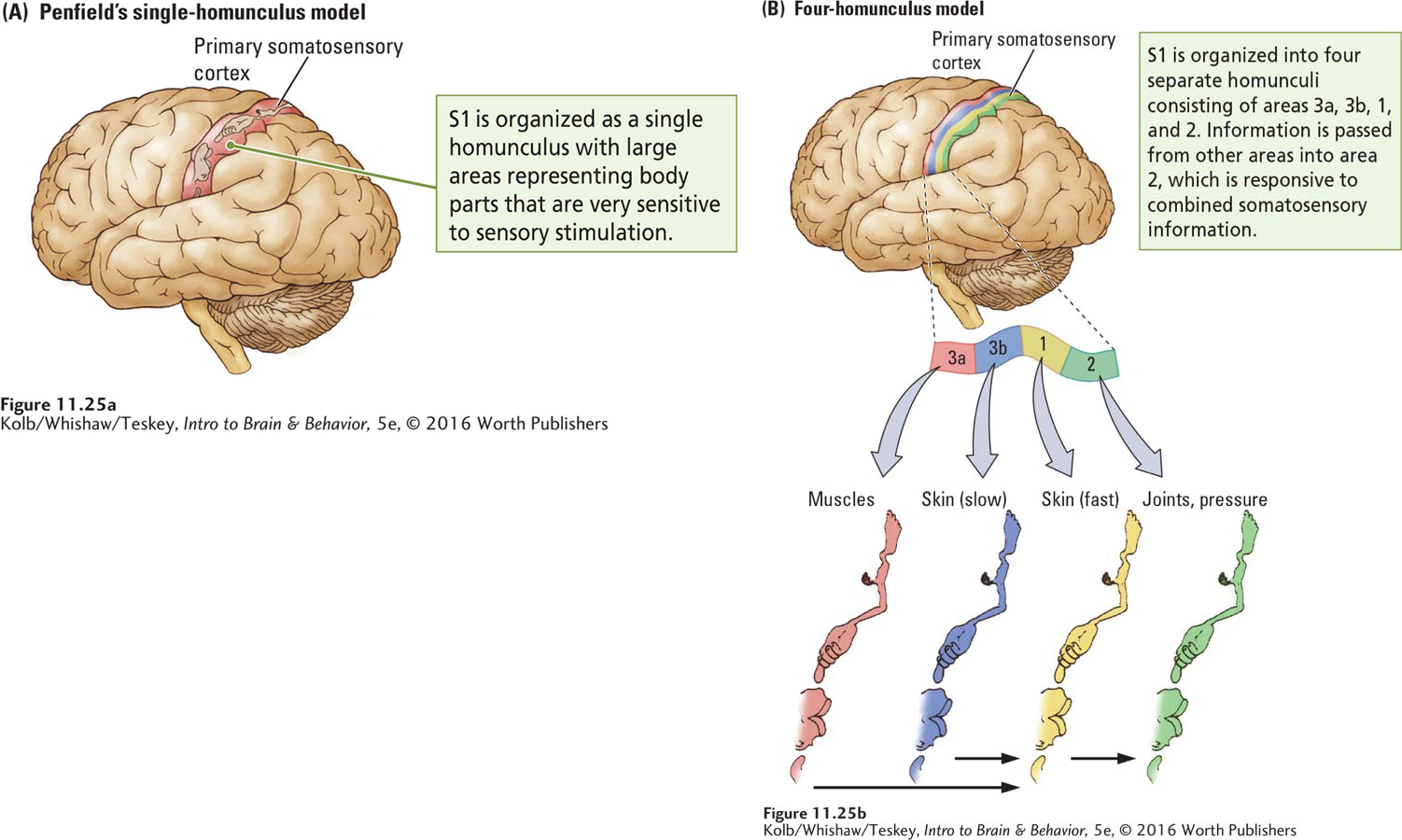
Using smaller electrodes and more precise recording techniques in monkeys, Jon Kaas (1987) found that Penfield’s homunculus could be subdivided into a series of smaller homunculi. When Kaas stimulated sensory receptors on the body and recorded the activity of cells in the sensory cortex, he found that the somatosensory cortex comprises four representations of the body. Each is associated with a class of sensory receptors.
The progression of these representations across S1 from front to back is shown in Figure 11-25B. Area 3a cells are responsive to muscle receptors; area 3b cells are responsive to slow-
391
Perceptions constructed from elementary sensations depend on combining the sensations. This combining takes place as areas 3a and 3b project onto area 1, which in turn projects onto area 2. Whereas a cell in area 3a or 3b may respond to activity in only a certain area on a certain finger, for example, cells in area 1 may respond to similar information from a number of fingers.
At the next level of synthesis, cells in area 2 respond to stimulation in a number of locations on a number of fingers as well as to stimulation from different kinds of somatosensory receptors. Thus, area 2 contains multimodal neurons responsive to force, orientation, and direction of movement. We perceive all these properties when we hold an object in our hands and manipulate it.
See Section 9-1 for details on receptive fields.
With each successive information relay, both the size of the pertinent receptive fields and the synthesis of somatosensory modalities increase. The segregation of sensory neuron types at the level of the cortex is likely the basis for our ability to distinguish among different kinds of sensory stimuli coming from different sources. For example, we distinguish between tactile stimulation on the surface of the skin, which is usually produced by some external agent, and stimulation coming from muscles, tendons, and joints, which is usually produced by our own movements.
At the same time, we perceive the combined sensory properties of a stimulus. For instance, when we manipulate an object, we know the object both by its sensory properties, such as temperature and texture, and by the movements we make as we handle it. Thus, the cortex provides for somatosensory synthesis too. The tickle sensation seems rooted in an other-
Figure 9-33 shows functional column organization in V1.
Research by Vernon Mountcastle (1978) shows that cells in the somatosensory cortex are arranged in functional columns running from layer I to layer VI, similar to the functional columns found in the visual cortex. Every cell in a functional somatosensory cortical column responds to a single class of receptors. Some columns are activated by rapidly adapting skin receptors, others by slowly adapting skin receptors, still others by pressure receptors, and so forth. All neurons in a functional column receive information from the same local skin area. In this way, neurons lying within a column seem to be an elementary functional unit of the somatosensory cortex.
392
RESEARCH FOCUS 11-6
Tickling
Everyone knows the effects and consequences of tickling. The perception is a curious mixture of pleasant and unpleasant sensations. The two kinds of tickling are kinismesis, the sensation from a light caress, and gargalesis, the pleasurable effect of hard rhythmic probing.
The tickle sensation is felt not only by humans but also by other primates and by cats, rats, and probably most mammals. Play in rats is associated with 50-
Tickling is rewarding because people and animals solicit tickles from others. They even enjoy observing others being tickled. Using a robot and brain imaging techniques, Sarah Blakemore and her colleagues (1998) explained why we cannot tickle ourselves.
Blakemore had participants deliver two kinds of identical tactile stimuli to the palms of their hand. In one condition, the stimulus was predictable and in the other a robot introduced an unpredictable delay in the stimulus. Only the unpredictable stimulus was perceived as a tickle. Thus, it is not the stimulation itself but its unpredictability that accounts for the tickle perception. This is why we cannot tickle ourselves. Yet Windt and associates (2015), using a self-
One interesting feature of tickling is the distinctive laughter it evokes. This laughter can be identified by sonograms (sound analysis), and people can distinguish tickle-
Intrigued by findings that all apes appear to laugh during tickling, Ross and coworkers (2009) compared tickle-

Effects of Somatosensory Cortex Damage
Damage to S1 impairs the ability to make even simple sensory discriminations and movements. Suzanne Corkin and her coworkers (1970) demonstrated this effect by examining patients with cortical lesions that included most of areas 3-
The tests included pressure sensitivity, two-
The results of other studies of both humans and other animals have shown that damage to the somatosensory cortex also impairs simple movements. For example, limb use in reaching for an object is impaired, as is the ability to shape the hand to hold an object (Leonard et al., 1991).
Section 7-7 considers debates over using laboratory animals in brain–
Nevertheless, like the motor cortex, the somatosensory cortex is plastic. Plasticity is illustrated by the reorganization of somatosensory cortex after deafferentation. In 1991, Tim Pons and his coworkers reported a dramatic change in the somatosensory maps of monkeys in which the ganglion cells for one arm had been cut, deafferentating the limb, a number of years earlier. The researchers had wanted to develop an animal model of damage to sensory nerves that could offer insight into human injuries, but they were interrupted by a legal dispute with an animal advocacy group. Years later, as the health of the animals declined, a court injunction allowed the mapping experiment to be conducted.
393
Pons and his coworkers discovered that the area of somatosensory cortex that had formerly represented the arm no longer did so. Light touches on a monkey’s lower face now activated cells in what had formerly been the cortical arm region. As illustrated in Figure 11-26, the cortical facial area had expanded by as much as 10 to 14 millimeters, virtually doubling its original size by entering the arm area.
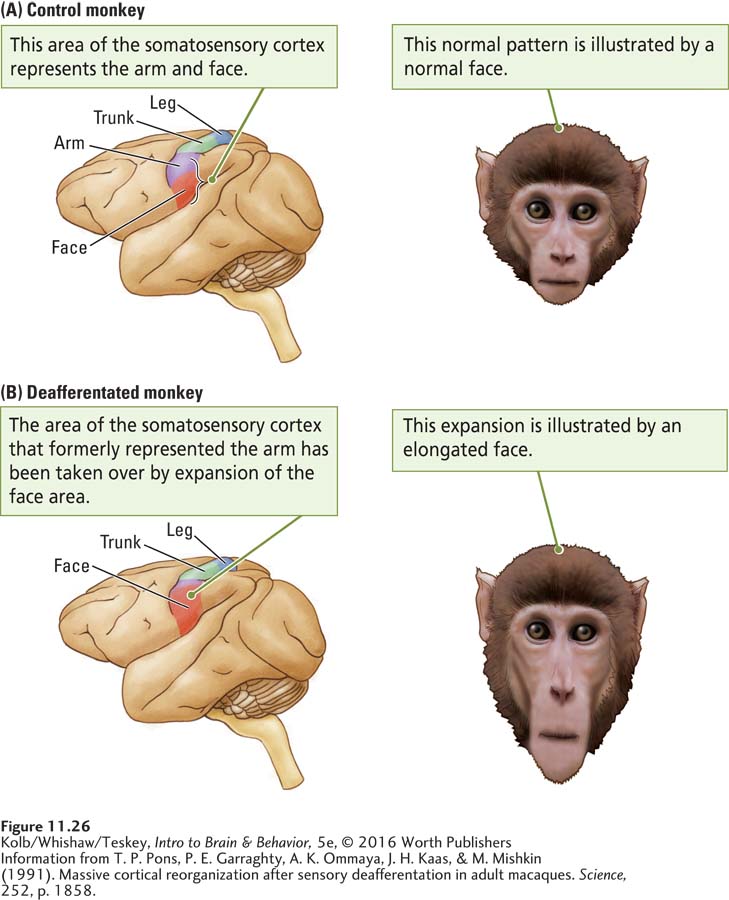
This massive change was completely unexpected. The stimulus–
Figure 14-23 diagrams this phenomenon.
What could account for this expansion of the face area into the arm area? There is evidence for preexisting axon collaterals that are not normally active, but these collaterals would probably not be able to extend far enough to account for all of the cortical reorganization. Another possibility is that within the thalamic and brainstem relay pathways, facial-
Somatosensory Cortex and Complex Movement
Apraxia derives from the Greek words for no and action.
How are our abilities to move and to interpret stimulation on the body related? Our somatosensory cortex actually makes an important contribution to movement. Damage to the secondary somatosensory cortex does not disrupt the plans for making movements, but it does disrupt how the movements are performed, leaving their execution fragmented and confused. This inability to complete a plan of action accurately—
394
A woman with a biparietal lesion [damage on the left-
The same patient also showed another unusual phenomenon that might possibly be apraxic in nature. She could never finish an undertaking. She would begin a job, drop it, start another, abandon that one, and within a short while would have four or five uncompleted tasks on her hands. This would cause her to do such inappropriate actions as putting the sugar bowl in the refrigerator, and the coffeepot inside the oven. (Critchley, 1953, pp. 158–
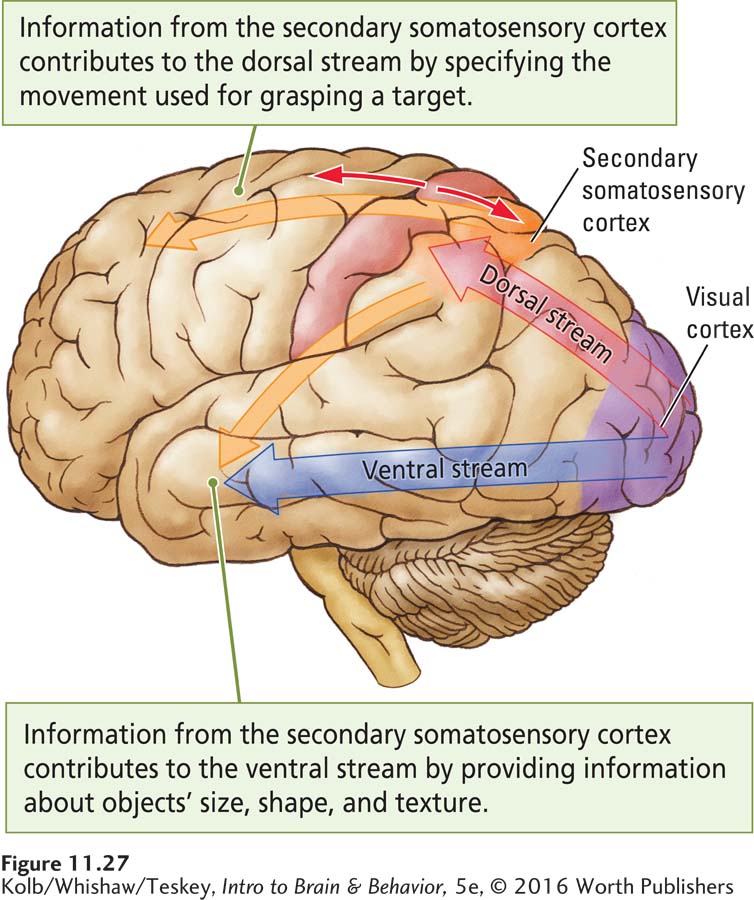
The somatosensory cortex contributes to movement by participating in both the dorsal and the ventral visual streams. The dorsal (how) stream, working without conscious awareness, provides vision for action, as when we automatically shape a hand as we reach to grasp a cup (recall Figure 11-1). Within this stream a number of channels specify the movements—
As Figure 11-27 illustrates, the dorsal visual stream projects to the secondary somatosensory cortex and then to the frontal cortex (Kaas et al., 2012). In this way, visual information is integrated with somatosensory information to produce unconscious movements appropriately shaped and directed to their targets, as in reaching for a cup. The secondary somatosensory area contributes perceptual information to the ventral stream by providing conscious haptic information about object identity and completed movements. From this information the frontal cortex can imagine the consequence of a movement and select the actions that appropriately follow from those already completed.
11-5 REVIEW
Exploring the Somatosensory Cortex
Before you continue, check your understanding.
Question 1
The ____________ somatosensory cortex, arranged as a series of homunculi, feeds information to the ____________ somatosensory cortex, which produces somatosensory perception.
Question 2
Damage to the secondary somatosensory cortex produces ____________, an inability to complete a series of movements.
Question 3
The somatosensory cortex provides information to the ____________ stream to produce unconscious movements and also provides information to the ____________ stream for conscious recognition of objects.
Question 4
Explain briefly what phantom limb pain tells us about the brain.
Answers appear in the Self Test section of the book.